It’s been estimated that over one lifetime, a heart will beat 2.5 to 3 billion times without stopping. Yet the mechanics of how the heart physically carries out this function flawlessly, without fail, minute after minute, remains poorly understood on a molecular level.
Debabrata Dutta, PhD, a postdoc in the lab of Roger Craig, PhD, and Raúl Padrón, PhD, professors of radiology, used cryogenic electron microscopy (cryo-EM) to reveal in precise detail the molecular structure of the main contractile component of human cardiac muscle known as the thick filament. Published in Nature, the research shows how the motor protein responsible for contraction—myosin—is organized molecularly in the filament. This unprecedented level of detail allows scientists to understand how mutations in this structure lead to heart failure and can potentially be used to design therapies to treat defects in this complex machine.
“Fixing the heart is like fixing any physical motor or machine; you need to understand how it’s assembled and how all the parts interact with each other in order to find the point of failure—or in this case, the fundamental cause of disease,” said Dr. Dutta.
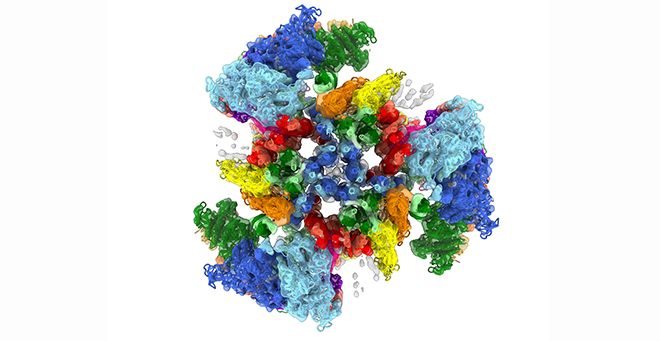
The heart beats because of interactions between myosin molecules in the thick filaments and actin in the thin filaments, which cause them to slide past each other to produce contraction. After each beat, the heart relaxes and fills with blood until the next contraction. Mutations in the thick filament can disrupt this perfectly timed machinery, causing cardiomyopathy, leading to heart failure.
“The thick filament myosin motors are constantly moving. Until now, the only images we were able to capture of them were like a blurry photograph; there wasn’t a whole lot of detail that we could make out,” explained Dr. Craig. “With the Medical School’s advanced cryo-EM facility, we’ve been able to produce images that are six times more detailed than anything previously reported.”
The thick filaments are made up of two proteins in addition to myosin, titin and cMyBP-C, all working together closely. The images produced by Dutta have revealed the extraordinarily complex organization and interactions that nobody had predicted. They show how the myosin motor heads, which generate contraction, are positioned in the filament via their elongated tails, which form the filament backbone.
The myosin tails define the architecture of the filament, dictated by binding to titin, which functions as a template, while interactions of the heads with cMyBP-C ensure full relaxation of the heart between beats. Even a slight misalignment in these structures can result in less efficiency or even heart failure.
According to Craig, knowing this architecture will help clinical researchers to address fixing it, with the hope that drugs can be designed to restore proper alignment and function.
A key component in achieving these insights was obtaining thick filament samples from human heart muscles. Most studies of the thick filament structure have come from invertebrates, which, it turns out, are far differently structured than vertebrate heart muscle.
Using heart muscle samples obtained from the lab of Kenneth S. Campbell, PhD, professor of cardiovascular medicine at the University of Kentucky College of Medicine, Dutta was able to isolate and purify thick filaments under conditions that correspond to the relaxation phase of the cardiac cycle.
Finding a solution for stabilizing the thick filaments for cryo-EM imaging took Dutta more than a year of trial and error. A key factor was the use of mavacamten, a drug recently approved by the FDA for the treatment of obstructive hypertrophic cardiomyopathy. This drug works by stabilizing the myosin heads so that cardiac muscle can relax better.
“Thanks to Dr. Dutta’s work and the resolving power of the cryo-EM images, we can make out how cMyBP-C binds to the thick filament and regulates the activity of the myosin heads. Now we can analyze that and other interactions that were previously hidden from our view,” said Padrón.