 |
|
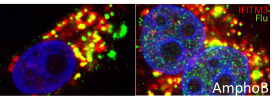
| This image shows a human cancer cell being infected by flu, in green, after being treated with Amphotericin B, at right, or without, at left. Invading the cell nuclei, in blue, is the flu’s goal. When Amphotericin B is added to the cell, the flu can invade the nulcei much more efficiently as seen on the right, even when IFITM3, in red, is present. |
Scientists at the UMass Medical School and the Wellcome Trust Sanger Institute have discovered evidence that a widely used antifungal medicine increases susceptibility to flu infection in mice and cell cultures. Published online in Cell Reports, the study shows that Amphotericin B, commonly given to cancer and bone marrow transplant patients to fight invasive fungal infections, neutralizes an important antiviral protein, making it easier for viruses to infect cells.
These findings suggest that patients taking the antifungal therapy may be functionally immunocompromised and vulnerable to influenza and potentially other viruses.
“While these studies don’t confirm that such an interaction may translate into clinical relevance for patients, it does suggest that some vigilance is warranted, especially for patients who are undergoing treatment for cancer and may already have suppressed immune systems,” said Abraham Brass, MD, PhD, assistant professor of microbiology & physiological systems and senior author of the study.
Paul Kellam, PhD, professor from the Wellcome Trust Sanger Institute and co-author of the study said, “This is an important discovery and the consequences for patients on certain antifungal treatments should now be investigated. Preventative flu vaccinations, rapid antiviral therapy or alternative antifungal treatment could be offered to these patients when at risk of flu infection.”
Found in nearly all human cells, IFITM3 works to alter the cell membrane, making it more difficult for viruses, such as the influenza virus, to penetrate the cell’s outer layer. When IFITM3 is inactive, influenza viruses can more readily infiltrate and infect the cell. Previous studies by Drs. Kellam and Brass and their colleagues have also shown that people who have a genetic variant in the IFITM3 gene are more susceptible to influenza.
Brass and his lab were working to understand how IFITM3 protects cells from viral infection when they discovered the link between Amphotericin B and influenza. “Several cell cultures in the lab became contaminated with a fungus,” said Brass. “We treated them with Amphotericin B, not knowing it would have an effect on IFITM3 activity. Surprisingly, when we tested for influenza infection we found no IFITM3 activity in the normal, wild type, cells. At that point, we began to suspect that Amphotericin B was having an effect on IFITM3.”
This unexpected revelation opened up a new line of inquiry that revealed that Amphotericin B was preventing IFITM3 from fending off the influenza virus. “When we treated lung cancer cells with the antifungal drug, we saw the antiviral protection normally provided by IFITM3 disappear,” said Christopher R. Chin, research associate in the Brass lab and co-first author of the study.
To better appreciate the effects Amphotericin B has on IFITM3, Brass teamed up with Kellam to treat mice with the antifungal drug. The pair found that once the mice contracted influenza, they displayed the same, more severe flu symptoms as mice completely lacking the protective IFITM3 gene. In the absence of the influenza virus, the mice treated with the Amphotericin B showed no signs of illness.
This research indicates that patients undergoing Amphotericin B antifungal treatments could potentially lose the protective effects of IFITM3, increasing the risk of flu infections in patients with already compromised immune systems.
“Sometimes a very useful drug can also have unforeseen effects,” said Brass. “We now see that a major part of the body’s natural defenses to influenza virus is rendered inactive by Amphotericin B in cells and mice. It’s our hope that reporting the consequences of this interaction may stimulate further translational studies and potentially guide patient care.”
Both Kellam and Brass agree that further work is now needed to evaluate whether this effect has any clinical significance for patients receiving Amphotericin-B-based treatments.
This research was part of an ongoing collaboration between institutes in the United States (UMass Medical School) and the United Kingdom (Wellcome Trust Sanger Institute).