 |
|
| Research by Gang Han, PhD, shows that upconversion nanoparticles that can convert near-infrared light into red light can be used to extend photodynamic therapy for some cancers found in deeper tissues. |
An international group of scientists led by Gang Han, PhD, has combined a new type of nanoparticle with an FDA-approved photodynamic therapy to effectively kill deep-set cancer cells in vivo with minimal damage to surrounding tissue and fewer side effects than chemotherapy. This promising new treatment strategy could expand the current use of photodynamic therapies to access deep-set cancer tumors.
“We are very excited at the potential for clinical practice using our enhanced red-emission nanoparticles combined with FDA-approved photodynamic drug therapy to kill malignant cells in deeper tumors,” said Dr. Han, lead author of the study and assistant professor of biochemistry & molecular pharmacology. “We have been able to do this with biocompatible low-power, deep-tissue-penetrating 980-nm near-infrared light.”
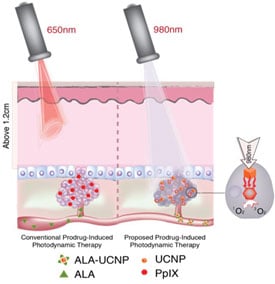
In photodynamic therapy, also known as PDT, the patient is given a non-toxic light-sensitive drug, which is absorbed by all the body’s cells, including the cancerous ones. Red laser lights specifically tuned to the drug molecules are then selectively turned on the tumor area. When the red light interacts with the photosensitive drug, it produces a highly reactive form of oxygen (singlet oxygen) that kills the malignant cancer cells while leaving most neighboring cells unharmed.
Because of the limited ability of the red light to penetrate tissue, however, current photodynamic therapies are only used for skin cancer or lesions in very shallow tissue. The ability to reach deeper set cancer cells could extend the use of photodynamic therapies.
In research published online by the journal ACS Nano of the American Chemical Society, Han and colleagues describe a novel strategy that makes use of a new class of upconverting nanoparticles (UCNPs), a billionth of a meter in size, which can act as a kind of relay station. These UCNPs are administered along with the photodynamic drug and convert deep penetrating near-infrared light into the visible red light that is needed in photodynamic therapies to activate the cancer-killing drug.
To achieve this light conversion, Han and colleagues engineered a UCNP to have better emissions in the red part of the spectrum by coating the nanoparticles with calcium fluoride and increasing the doping of the nanoparticles with ytterbium.
In their experiments, the researchers used the low-cost, FDA-approved photosensitizer drug aminolevulinic acid and combined it with the augmented red-emission UCNPs they had developed. Near-infrared light was then turned on the tumor location. Han and colleagues showed that the UCNPs successfully converted the near-infrared light into red light and activated the photodynamic drug at levels deeper than can be currently achieved with photodynamic therapy methods. Performed in both in vitro and with animal models, the combination therapy showed an improved destruction of the cancerous tumor using lower laser power.
Yong Zhang, PhD, chair professor of National University of Singapore and a leader in the development and application of upconversion nanoparticles, who was not involved in the study, said that by successfully engineering amplified red emissions in these nanoparticles, the research team has created the deepest-ever photodynamic therapy using an FDA-approved drug.
“This therapy has great promise as a noninvasive killer for malignant tumors that are beyond 1 cm in depth—breast cancer, lung cancer and colon cancer, for example—without the side-effects of chemotherapy,” Dr. Zhang said.
Han said, “This approach is an exciting new development for cancer treatment that is both effective and nontoxic, and it also opens up new opportunities for using the augmented red-emission nanoparticles in other photonic and biophotonic applications.”