Gene Therapy Vectors
What Are Gene Therapy Vectors?
Gene therapy vectors can be categorized into two classes: Artificial non-viral vectors and viral vectors. Artificial vectors possess several theoretical advantages over viral vectors, such as simple production methods that are easily scalable, and amenable to carrying large genes, and have low immunogenicity potential. However artificial vectors remain much less efficient than viral vectors for in vivo or ex vivo gene transfer and transgene expression is generally transient.
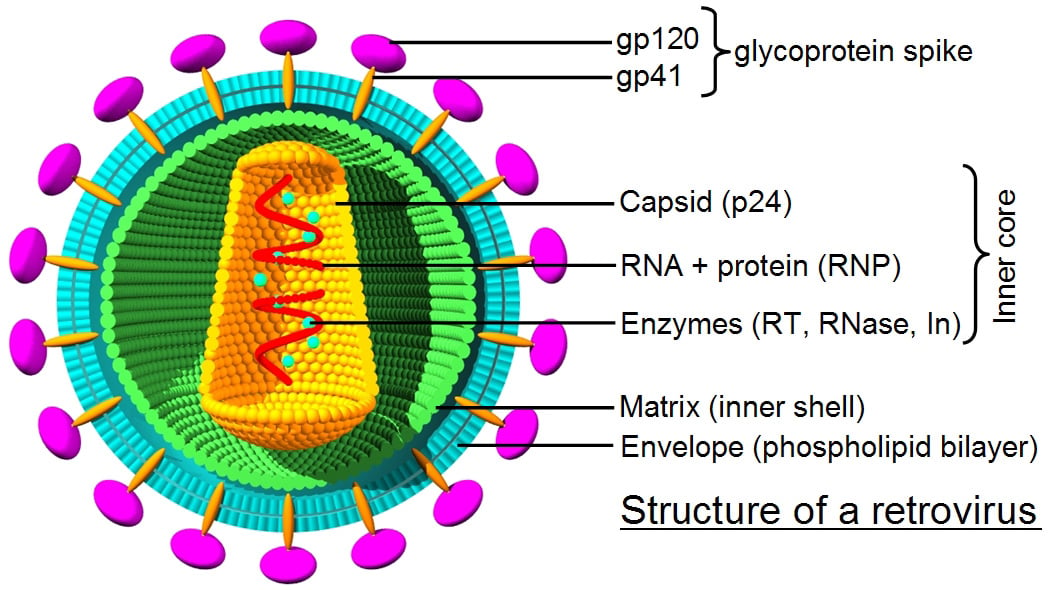 Over the years several viruses that infect mammals have been engineered to become naturally evolved gene delivery vehicles for gene therapy. The surface proteins on viral particles interact with their natural cell surface receptors, which triggers the cellular uptake process known as endocytosis. Once inside a target cell, wild-type viruses eventually deliver their genetic information to the nucleus to start the cycle of viral replication through expression of viral genes.
Over the years several viruses that infect mammals have been engineered to become naturally evolved gene delivery vehicles for gene therapy. The surface proteins on viral particles interact with their natural cell surface receptors, which triggers the cellular uptake process known as endocytosis. Once inside a target cell, wild-type viruses eventually deliver their genetic information to the nucleus to start the cycle of viral replication through expression of viral genes.
The process of transforming wild-type viruses into recombinant viral vectors consists in replacing all or most viral genes with a therapeutic gene cassette while retaining signal sequences necessary for replication and packaging. Recombinant viral vectors are manufactured in producer cell lines supplemented in trans with viral proteins necessary for replication, capsid and envelope generation. Vectors based on gamma retrovirus, lentivirus, adenovirus (AdV), adeno-associated virus (AAV) and herpes simplex virus (HSV) are among the most widely used viral vectors in current gene therapy studies, and are more advanced in clinical translation than other types of viral vectors.
Gamma retrovirus and lentivirus are RNA viruses that use a reverse transcriptase and integrase to insert their proviral complementary DNA into the host genome. Integration into host genome, the distinctive feature of retroviral vectors, is a double-edged sword when it comes to gene therapy. Genomic integration ensures transmission of the vector transgene cassette and its expression in the progeny, but its randomness carries a risk of insertional mutagenesis by potentially disrupting tumor suppressor genes or activating oncogenes.
AdV has a DNA genome that resides in the host cell nucleus as an episome, and as such insertional mutagenesis is unlikely. AdV is able to transduce a broad range of quiescent and proliferating human cells including tumor cells, making it an attractive vector for the development of anti-tumor gene therapy strategies. However, in vivo administration of AdV triggers strong immune responses, which preclude their use for gene therapy applications in genetic diseases where long-term gene expression may be necessary. High-capacity AdV vectors are the exception as they do not carry any viral genes and have been shown to mediate long-term gene expression in vivo.
Adeno-Associated Viruses (AAV)
AAV is a small single-stranded DNA virus that depends on other viruses for replication and packaging. Importantly AAV is nonpathogenic. Recombinant AAV (rAAV) vectors carrying inverted terminal repeats as the only viral component entered the gene therapy arena much later than retroviral and AdV vectors, but have quickly gained popularity due to their broad tissue tropism, exceptional in vivo gene transfer efficiency and sustained transgene expression, as well as a proven safety profile in humans. In 2012, the first commercial product for human gene therapy Glybera, a rAAV vector expressing lipoprotein lipase (LPL) that treats LPL deficiency, obtained official approval in Europe, representing a milestone in rAAV gene therapy.
HSV is a naturally neurotropic virus. After initial infection in skin or mucosal membranes, HSV is taken up by sensory nerve terminals, travels retrogradely to neuronal cell bodies, and delivers its DNA genome into the nucleus where it establishes a state of latency. Environmental stimuli trigger its replication and release at the nerve terminal where it infects and replicates rapidly in skin cells generating the common cold sore. The natural neurotropism of HSV vectors make them well suited for gene therapy of neurological disorders. In addition replication-conditional HSV vectors have proven to be exceptionally efficient oncolytic agents.
Our Work At GTC
AAV is the primary gene therapy platform driving the GTC Research. The GTC is a world leader in novel AAV discovery, vector development, production, preclinical and clinical gene therapy research. Among our faculty Dr. Flotte was the first to translate rAAV gene therapy to patients (Flotte 1995) and Dr. Gao pioneered and led the discovery of novel AAV serotypes from primate origin (Gao 2002). We develop gene therapy strategies to treat a broad range of diseases, which impact on both the central nervous system and peripheral organs, such as Tay-Sachs disease and GM1 gangliosidosis (Sena-Esteves Lab), Canavan disease (Gao Lab), amyotrophic lateral sclerosis (Mueller, Sena-Esteves and Gao Labs), Huntington’s disease (Mueller, Sena-Esteves, and Gao Labs), retinal disorders such as Retinitis Pigmentosa and Ciliopathies (Punzo and Khanna Labs), Pompe disease (ElMallah Lab), cystic fibrosis (Flotte Lab), and alpha-1 anti-trypsin deficiency (Flotte and Mueller Labs). To move gene therapy towards clinical use, we conduct clinical trials of our gene therapy strategies (Flotte Lab), study the safety profile of gene delivery technology (Gao, Mueller, Flotte and Sena-Esteves Labs), and develop more efficacious rAAV vectors for human use (Sena-Esteves and Gao Labs).
Learn more about the breakthrough research at the Horae GTC
