Fatty Oxidation Disorders
Genetic disorders of mitochondrial fatty acid oxidation (FAO) represent a relatively common class of metabolic disorders, exceeding more than 1 in 15,000 newborns. These disorders are associated with genes involved in the mitochondrial b-oxidation of fatty acids, which provide fuel once glycogen stores are depleted during prolonged fasting or during times of increased energy demands and physiological stress. During these circumstances liberation from triglycleride stores in adipose tissue causes free fatty acids circulate to the liver and striated muscle. After the fatty acids diffuse (short chain) or are actively transported (long chain) across the cellular and mitochondrial membranes, the oxidation of fatty acids occurs within the mitochondrial matrix. b-oxidation proceeds in a cyclical fashion resulting in the removal of sequential 2-carbon units as acetyl-CoA, for entry into the TCA cycle
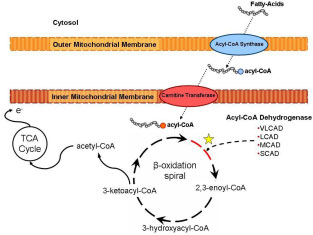 Importantly, the first step of each cycle in the mitochondrial matrix is catalyzed by an acyl-CoA dehydrogenase. This rate limiting function is performed by a family of enzymes that differ in their substrate specificity based on the carbon chain length of the acyl CoA molecule. The acyl-CoA dehydrogenases (ACDs) are a family of 5 mitochondrial enzymes involved in fatty acid and amino acid metabolism that catalyze the transfer of electrons from various acyl-CoA esters to electron transfer flavoprotein. Very long, medium and short chain acyl-CoA dehydrogenases (VLCAD, MCAD and SCAD) catalyze the first step in the b-oxidation cycle with substrate specificities of 16-, 8- and 4- carbon chains, respectively.
Importantly, the first step of each cycle in the mitochondrial matrix is catalyzed by an acyl-CoA dehydrogenase. This rate limiting function is performed by a family of enzymes that differ in their substrate specificity based on the carbon chain length of the acyl CoA molecule. The acyl-CoA dehydrogenases (ACDs) are a family of 5 mitochondrial enzymes involved in fatty acid and amino acid metabolism that catalyze the transfer of electrons from various acyl-CoA esters to electron transfer flavoprotein. Very long, medium and short chain acyl-CoA dehydrogenases (VLCAD, MCAD and SCAD) catalyze the first step in the b-oxidation cycle with substrate specificities of 16-, 8- and 4- carbon chains, respectively.
Currently our lab is applying rAAV-based transduction of skeletal muscle for those FAO deficiencies that are most common in humans, medium chain acyl CoA dehydrogenase (MCAD) and very long chain acyl CoA dehydrodgenase (VLCAD). Over the past 5 years, mouse models have become available for each of these disorders, and mass newborn screening for FAO disorders has been initiated in many states. Our prior work has relied heavily on rAAV1 pseudotyped vectors for skeletal muscle transduction. The emergence of rAAV9-based vectors now provides the potential for even greater efficiency in using transduction of skeletal muscle and cardiac muscle as a platform for gene therapy of systemic genetic diseases. We are investigating to compare rAAV1 and rAAV9 in the context of a translational application to VLCAD and MCAD deficiencies. Specifically, the feasibility of molecular and biochemical correction of VLCAD and MCAD deficiencies is tested in mouse models of these deficiencies.
Our primary focus is on determining whether rAAV9 (vs. rAAV1)-mediated transduction of skeletal and cardiac muscle will result in greater improvement of acyl carnitine profiles (i.e., clearance of abnormally high levels of fatty acyl carnitine metabolites that are characteristic of this disorder) and MRS profiles. We are also going to develop formal preclinical toxicology studies of muscle delivery of rAAV9 or rAAV1-VLCAD and rAAV9 or rAAV1-MCAD which will be completed in anticipation of new phase I clinical trials of gene therapy for these FAO disorders.
