Programming gene expression and cellular functions with synthetic RNA switches
RNA switches, structured small noncoding RNA domains that control gene expression independent of any protein factors, are a class of very useful tools for exogenous, precise control of gene expression. They not only can be used in genetics studies for spatial & temporal manipulation of endogenous genes, but also can be used in vectored gene therapies for temporal or dosing control of therapeutic transgene expression, or in cell-based therapies for precise programming of engineered-cell functions. RNA switch thus represents a very promising platform technology that has the potential to enable future gene and cell therapy application to diverse diseases that can't be safely or effectively treated with the current gene or cell therapy technologies (learn more here).
Our group creates useful RNA switches and develops RNA switch-regulated gene therapies for diverse disease applications. A body of our prior studies have moved artificial RNA switches from working efficiently in vitro to now functioning with wide dynamic ranges in animals. These studies also established a strong proof of concept that RNA switches could enable safer and more effective applications and expand the use of in vivo gene therapies to broader disease indications.
We are now very interested in the following three directions:
- Tool development: engineering parts (novel RNA switches, other RNA-based tools, therapeutic payloads) for the development of programmable gene therapies.
- Mechanistic studies: investigating how the in-house developed RNA switches work in mammalian cells.
- Gene therapy applications: developing programmable gene and cell-based therapies for fatal genetic diseases (e.g. Duchenne muscular dystrophy, Rett Syndrome), metabolic disorders (e.g. diabetes, NASH), infectious diseases (e.g. HIV infection, hepatitis B, COVID-19), and cancers.
We are always interested in possible collaborations with researchers who focus on the biology of specific diseases or novel therapeutic targets where regulatable gene therapy technology are useful.
Ongoing projects in the lab
RNA switch-regulated gene therapy technologies for long-term, tunable expression of short-lived therapeutic proteins
Long-term delivery of short-lived therapeutic proteins are generally desirable for the treatment of diverse conditions ranging from diabetes, to non-alcoholic steatohepatitis (NASH), to obesity. AAV-delivered in vivo gene therapy can turn transduced somatic cells (e.g. skeletal muscle cells) into a bio-factory to support long-lasting (years to decades) production of secreted therapeutic proteins. RNA switch-regulated protein gene therapy, which utilizes AAV transduced somatic cells to continuously produce and secrete protein therapeutics in their very native forms into the bloodstream, is therefore an attractive and generalizable approach to long-term delivery of short-lived proteins for a wide range of diseases that require chronic use of protein-based pharmacotherapies.
We have previously developed a self-cleaving ribozyme-based RNA on switch system that efficiently regulates AAV transgene expression with up to 200-fold dynamic range in mouse (Zhong et al. Nature Biotechnology. 2020). In this system, an in-house optimized, self-cleaving hammerhead ribozyme T3H38 is built in the 3ʹ-untranslated region (UTR) of an AAV transgene. During transcription, this ribozyme cleaves itself in cis with high specificity, and its enzymatic activity depends solely on its RNA conformation, and not on any protein cofactor. This ribozyme thus keeps the transgene in the "OFF" state through self-cleavage-mediated specific disruption of transgene mRNA. A steric-blocking antisense oligonucleotide (ASO) v-M8, which is complementary to the ribozyme and provided in trans, could block the ribozyme’s self-cleavage and thus switch on and tune the expression of the transgene. Utilizing this T3H38/v-M8 RNA switch system, we achieved in vivo regulation of AAV-mediated luciferase expression over a period of 43 weeks and precise control of AAV-mediated expression of erythropoietin to physiological level in mice. This study provides a proof of concept that RNA switches could expand the use of AAV gene therapy to some major common diseases.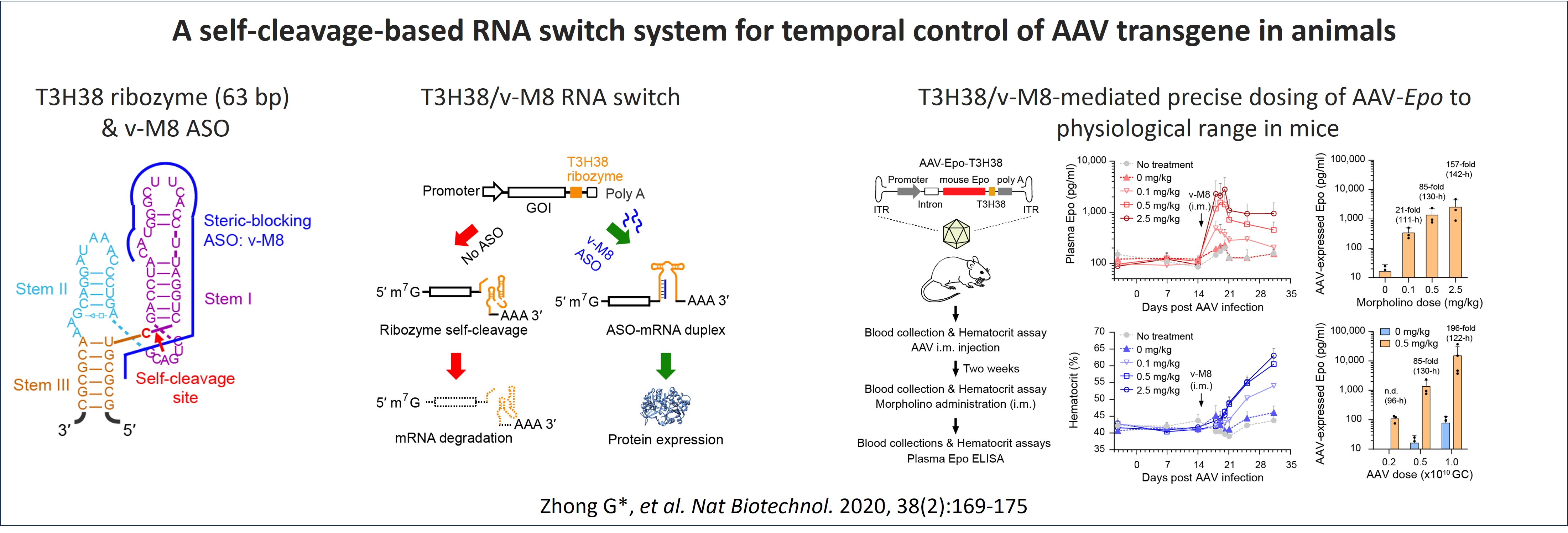
On the basis of this study, our lab has been developing and optimizing modular and generalizable RNA switch systems and utilizing our switch system to develop tunable AAV gene therapies. More details will be shared here soon.
RNA switch-regulated AAV-CRISPR/Cas systems for in vivo inducible gene editing therapies
AAV-delivered extrahepatic genome editing therapy has enormous potential for treating a range of human diseases. However, a single dose AAV delivered to non-dividing cells could provide persistent transgene expression for years to decades, which could lead to multiple major safety concerns including (i) persistent off-target editing of the genome, (ii) persistent immune response against nonself-editor-expressing cells, and (iii) double strand break-associated insertion of AAV DNA into chromosomes. These issues could significantly increase the risk, undercut the efficacy, and limit the application of this approach. Genetic switch systems that can provide temporarily induced expression of a genome editor could help mitigate all these above issues. An ideal genetic switch for this purpose should have the following key features: (i) small-sized; (ii) non-immunogenic; (iii) leak-free when the switch is in its dormant state; and (iv) efficiently inducible. RNA-based cis-acting switches, with their small size (~100 bp) and lack of dependence on exogenous proteins, naturally meet the first two criteria, making ideal candidate switch systems for AAV-delivered in vivo genome editing therapies. The challenge is to develop RNA switches that also meet the remaining two criteria.
Through extensive RNA-switch engineering, we have now developed a multilayer-regulated CRISPR-Cas system that is leak-free, efficiently inducible, and compact enough to be packaged into a single AAV vector. In this system, the Cas protein expression, crRNA expression, crRNA folding, and crRNA maturation are simultaneously silenced by a built-in cis-acting RNA switch. The four-layer regulations can be simultaneously deactivated with a single steric-blocking antisense oligo (ASO) complementary to the cis-acting RNA. We have demonstrated in cell culture as well as in mice that this inducible CRISPR-Cas system has no or minimal leaky editing activity before induction, and can be efficiently switched “ON” by a single dose ASO. This study lays the foundation for developing safer and effective in vivo gene-editing platform technologies for treating diseases of muscle or CNS origin. More details will be shared here soon.
RNA switch-regulated AAV-receptor-decoy as a long-lasting vaccine-like anti-viral prophylaxis for high-risk individuals
Anti-COVID: The continuous emergence of SARS-CoV-2 variants with increased immune evasion capabilities presents a great challenge on developing a long-lasting COVID-19 prophylaxis. ACE2-Ig, a recombinant fusion protein of human ACE2 ectodomain with antibody Fc, can function as an ACE2 decoy to neutralize diverse SARS-CoV-2 variants, as well as a wide range of other coronaviruses that also utilize ACE2 as an essential receptor. AAV-delivered terminable ACE2-Ig gene therapy that utilize transduced somatic cells as a bio-factory to contineously produce and secrete ACE2-Ig into bloodstream is therefore a promising pathway to a long-lasting vaccine-like prophylaxis against diverse ACE2-utilizing coronaviruses of pandemic potential, for high-risk (e.g. the elderly and immunocompromised) individuals. Since the outbreak of the COVID-19 pandemic, our lab has been studying cross-species receptor usage of multiple coronaviruses and have developed two potent ACE2-Ig proteins, ACE2-Ig-95 and ACE2-Ig-105/106. These two proteins showed potent and robust neutralization activities against diverse SARS-CoV-2 variants including Omicron, with an average IC50 of up to 37 pM, in pseudovirus neutralization assays. In a stringent lethal SARS-CoV-2 infection mouse model, therapeutic use of either of the ACE2-Ig proteins markedly lowered lung viral load by up to ~1000 fold, prevented the emergence of clinical signs in >75% animals, and increased animal survival rate from 0% (untreated) to >87.5% (treated; Lu et al. Microbiology Spectrum. 2023). ACE2-Ig-95 and ACE2-Ig-105/106 are therefore good lead payload molecules for a terminable, AAV-based anti-coronavirus prophylaxis.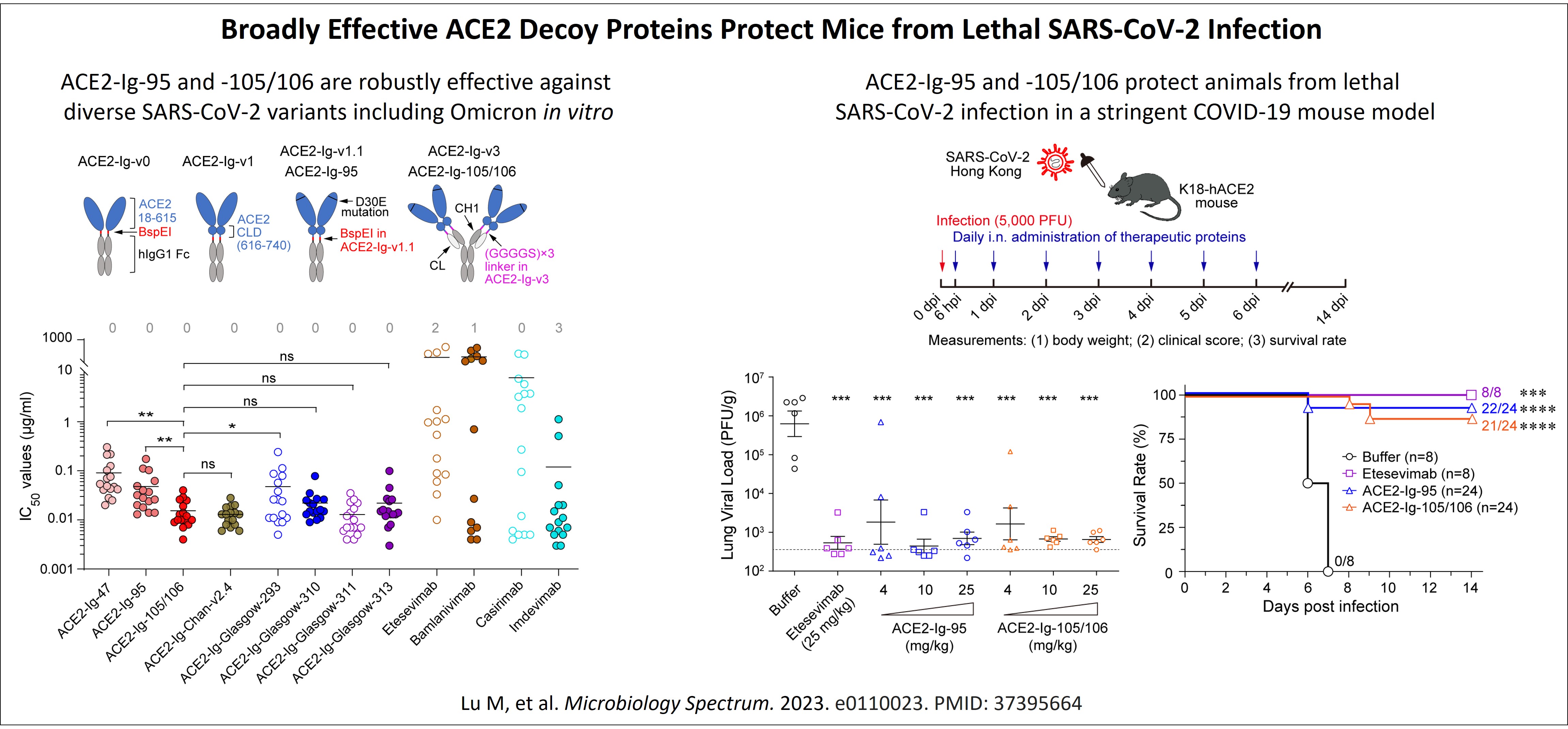
Anti-HIV-1: Effort on developing terminable AAV-delivered broadly neutralizing antibody-like molecule as a vaccine-like prophylaxis against HIV-1 is ongoing. Details will be shared here as soon as we can.
Projects in the pipeline. There are also some very promising projects that are too early for us to share yet. Details will be posted here as soon as we can.