Development of chemical probes to study STING biology
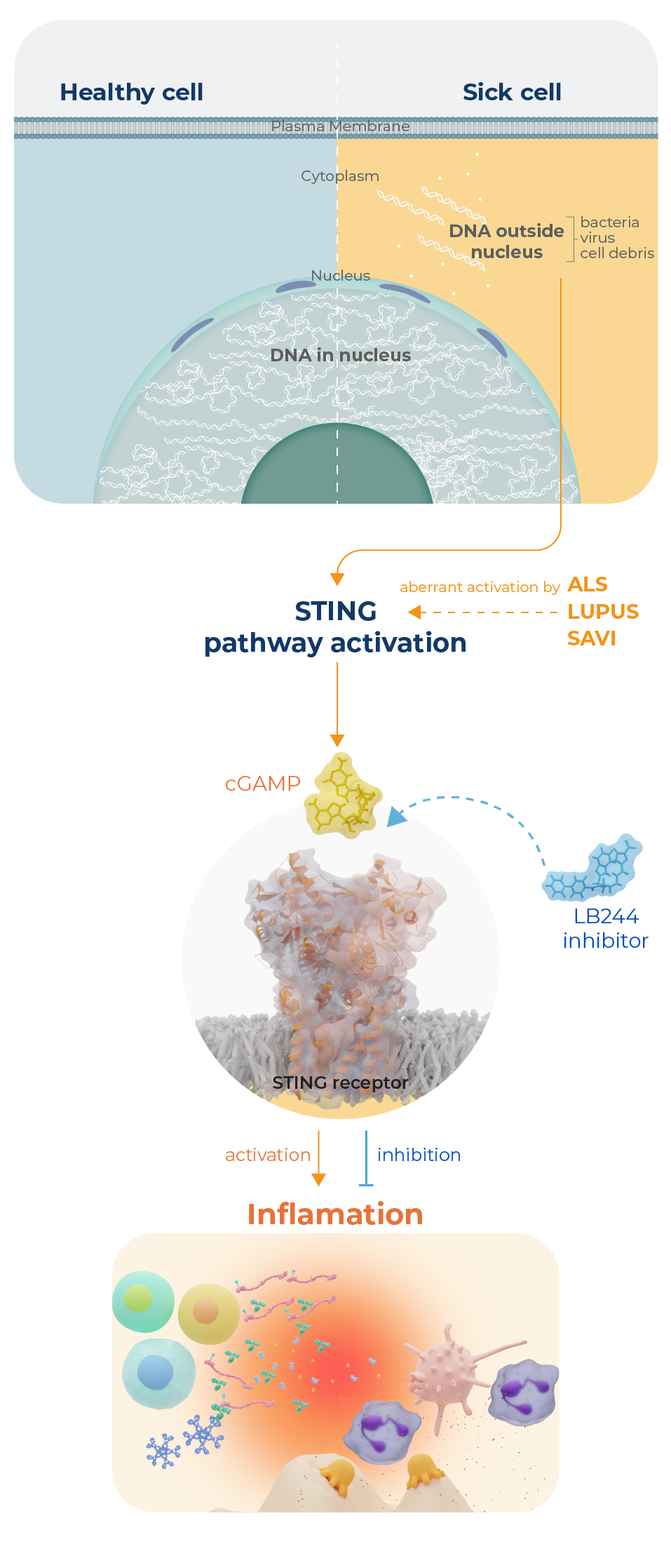
STING plays a crucial role in sensing cytosolic double strand DNA (dsDNA). Under normal conditions, dsDNA is restricted to the nucleus. However, upon infection with viruses or bacteria, dsDNA can been found in the cytosol. When present in the cytosol, dsDNA interacts with and allosterically activates an enzyme called cyclic GMP-AMP synthase or cGAS. cGAS binds one molecule of ATP and one molecule of GTP and catalyzes the generation of cyclic GAMP or cGAMP. cGAMP then binds to STING and triggers a series of events that leads to the increased expression of type I interferons which upregulate the immune system. In the context of viral or bacterial infections, this signaling pathway is beneficial. However, in various diseases (e.g., lupus, ALS, cancer) this signaling pathway is aberrantly regulated and the accumulation of cytosolic dsDNA leads to the overactivation of the immune system. Notably, STING knock out mice show decreased disease severity in multiple murine models of lupus and ALS. Given the therapeutic potential of a STING inhibitor, the Thompson lab, in collaboration with the Fitzgerald lab, has been focused on generating STING inhibitors. Notably, we first found that a PAD inhibitor could also inhibit STING. Using this compound as a lead, we then generated STING specific inhibitors that block STING signaling through a novel mechanism. Current efforts are focused on developing STING inhibitors with improved potency and selectivity as well as understanding how these compounds work in cells.
