Research
Insulin/IGF1 signaling (IIS) in breast cancer progression
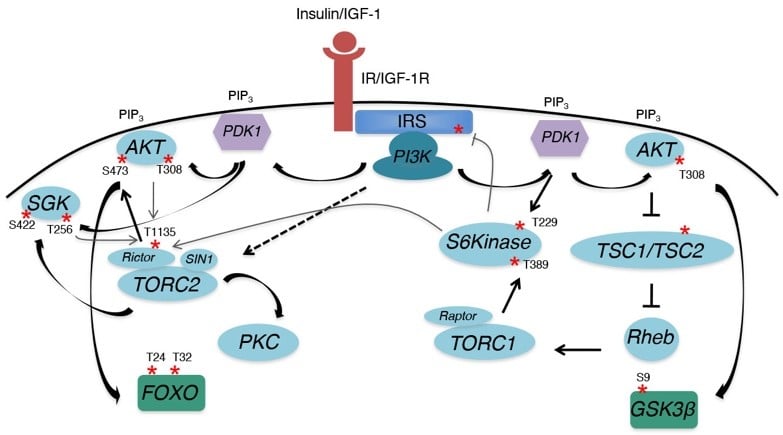 Obesity, a growing health epidemic, is associated with increased risk of both developing and dying from breast cancer. A major consequence of obesity is insulin resistance, leading to hyperinsulinemia. Although several factors have been implicated in contributing to the increased cancer risk due to obesity, hyperinsulinemia is an independent risk factor for breast cancer initiation and recurrence. Elevated insulin levels, which drive elevated IGF-1 levels as well, have also been implicated in tumor drug resistance, including resistance to PI3K targeted inhibitors and hormonal therapy. Both the insulin receptor (IR) and the closely related insulin-like growth factor-1 receptor (IGF-1R) have been implicated in breast cancer initiation and progression, but targeting the IIS pathway at the level of ligands and receptors has not been effective, due to adverse metabolic side-affects and feedback upregulation of pathway activity. We aim to develop a greater understanding of downstream signaling through the IR/IGF-1R to determine how to selectively target this essential signaling pathway in cancer without interfering with its role in normal metabolic homeostasis. In this regard, our research focuses on the insulin receptor substrate (IRS) adaptor proteins, essential signaling intermediates of the IIS pathway, as potential translational targets.
Obesity, a growing health epidemic, is associated with increased risk of both developing and dying from breast cancer. A major consequence of obesity is insulin resistance, leading to hyperinsulinemia. Although several factors have been implicated in contributing to the increased cancer risk due to obesity, hyperinsulinemia is an independent risk factor for breast cancer initiation and recurrence. Elevated insulin levels, which drive elevated IGF-1 levels as well, have also been implicated in tumor drug resistance, including resistance to PI3K targeted inhibitors and hormonal therapy. Both the insulin receptor (IR) and the closely related insulin-like growth factor-1 receptor (IGF-1R) have been implicated in breast cancer initiation and progression, but targeting the IIS pathway at the level of ligands and receptors has not been effective, due to adverse metabolic side-affects and feedback upregulation of pathway activity. We aim to develop a greater understanding of downstream signaling through the IR/IGF-1R to determine how to selectively target this essential signaling pathway in cancer without interfering with its role in normal metabolic homeostasis. In this regard, our research focuses on the insulin receptor substrate (IRS) adaptor proteins, essential signaling intermediates of the IIS pathway, as potential translational targets.
Insulin Receptor Substrate (IRS) protein function
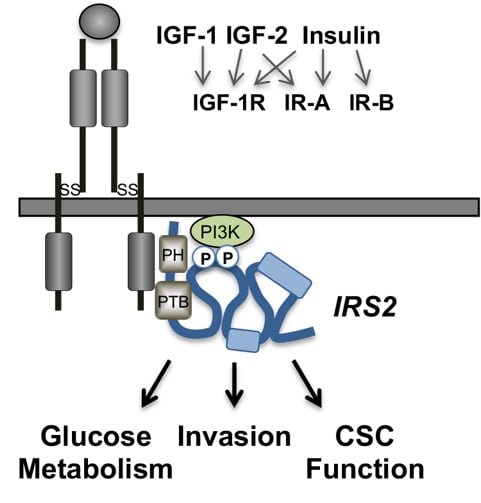 The IRS proteins are cytoplasmic adaptor proteins that mediate signals from the IR and IGF-1R to regulate organismal growth and metabolic homeostasis. Our in vivo studies to investigate the function of IRS-1 and IRS-2 in mammary tumor development and progression to metastasis revealed that IRS-1 and IRS-2 play divergent roles in mammary tumor metastasis. Mammary tumors that lack IRS-2 are significantly diminished in their ability to metastasize and tumors lacking IRS-1, and expressing only IRS-2, have enhanced metastatic potential. Our research has been directed toward understanding the molecular mechanisms by which these homologous proteins differentially impact breast cancer progression. Our studies have revealed that IRS-2 plays a dominant role in tumor progression through its involvement in regulating tumor cell invasion, cancer stem cell (CSC) function and tumor metabolism. The ability of IRS2 to regulate these functions is dependent upon upstream IR/IGF-1R activation and the recruitment and activation of PI3K. Additional regions of IRS2 also play essential roles in its function. Specifically, we identified a region within the IRS2 C-terminal tail that is both necessary and sufficient for promoting tumor cell invasion. Importantly, this “INV region” is not required for the IRS2-dependent regulation of glucose uptake, providing evidence that IRS functions important for cancer progression can be disrupted while preserving the normal metabolic functions of these adaptor proteins. Ongoing research in the lab focuses on investigating the mechanistic basis for differential IRS contributions to IR/IGF-1R signaling and tumor progression using cellular, biochemical, structural and molecular approaches. The translational goal of our work is to develop approaches to target IRS function/expression for therapeutic benefit.
The IRS proteins are cytoplasmic adaptor proteins that mediate signals from the IR and IGF-1R to regulate organismal growth and metabolic homeostasis. Our in vivo studies to investigate the function of IRS-1 and IRS-2 in mammary tumor development and progression to metastasis revealed that IRS-1 and IRS-2 play divergent roles in mammary tumor metastasis. Mammary tumors that lack IRS-2 are significantly diminished in their ability to metastasize and tumors lacking IRS-1, and expressing only IRS-2, have enhanced metastatic potential. Our research has been directed toward understanding the molecular mechanisms by which these homologous proteins differentially impact breast cancer progression. Our studies have revealed that IRS-2 plays a dominant role in tumor progression through its involvement in regulating tumor cell invasion, cancer stem cell (CSC) function and tumor metabolism. The ability of IRS2 to regulate these functions is dependent upon upstream IR/IGF-1R activation and the recruitment and activation of PI3K. Additional regions of IRS2 also play essential roles in its function. Specifically, we identified a region within the IRS2 C-terminal tail that is both necessary and sufficient for promoting tumor cell invasion. Importantly, this “INV region” is not required for the IRS2-dependent regulation of glucose uptake, providing evidence that IRS functions important for cancer progression can be disrupted while preserving the normal metabolic functions of these adaptor proteins. Ongoing research in the lab focuses on investigating the mechanistic basis for differential IRS contributions to IR/IGF-1R signaling and tumor progression using cellular, biochemical, structural and molecular approaches. The translational goal of our work is to develop approaches to target IRS function/expression for therapeutic benefit.
IRS2 and mitotic regulation in breast cancer
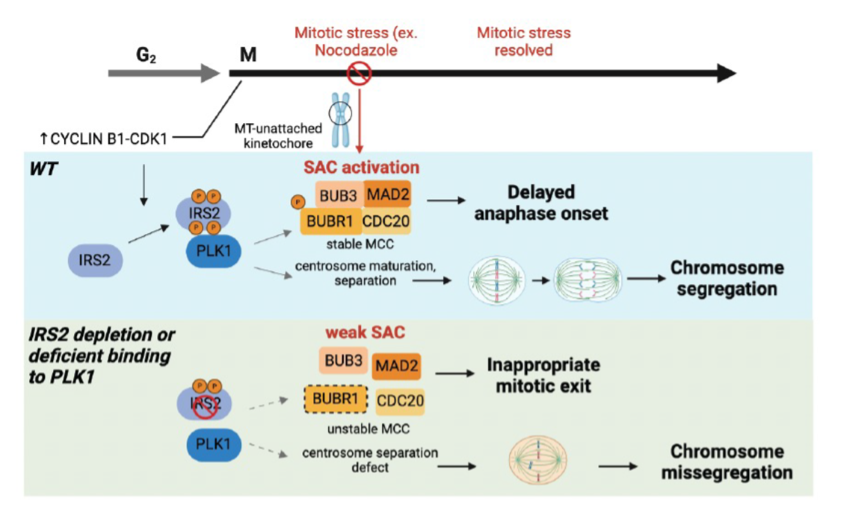 Independent of its function as an adaptor protein for the IIS pathway, IRS2 is involved in the regulation of the spindle assembly checkpoint (SAC) during mitosis. Our recent studies demonstrate that IRS2 is phosphorylated in a CDK1-manner as cells enter mitosis and this phosphorylation promotes interaction with and activation of the mitotic regulatory kinase PLK1. The interaction of IRS2 and PLK1 stabilizes BUBR1, an essential component of the SAC, to maintain arrest in cells exposed to mitotic stress until chromosomes fully attach to the microtubule spindle. In cells that lack IRS2 or express a PLK1-binding deficient mutant, centrosome separation is impaired and mitotic duration is shortened when cells are exposed to mitotic stress, leading to in an increased loss of cell viability. Triple Negative Breast Cancer (TNBC) is characterized by high chromosome instability and high proliferation rates and as a result, TN tumor cells require a strong SAC to respond to mitotic stress. The fact that IRS2 expression is elevated in TNBC and that it contributes to the regulation of the SAC and tumor cell viability in response to mitotic stress identifies a potential IRS2-specific vulnerability that could be exploited therapeutically in this breast cancer subtype. We are continuing to study the mechanism of IRS2 function in mitosis and the consequences of its loss on tumor response to chemotherapeutic drugs that induce mitotic stress.
Independent of its function as an adaptor protein for the IIS pathway, IRS2 is involved in the regulation of the spindle assembly checkpoint (SAC) during mitosis. Our recent studies demonstrate that IRS2 is phosphorylated in a CDK1-manner as cells enter mitosis and this phosphorylation promotes interaction with and activation of the mitotic regulatory kinase PLK1. The interaction of IRS2 and PLK1 stabilizes BUBR1, an essential component of the SAC, to maintain arrest in cells exposed to mitotic stress until chromosomes fully attach to the microtubule spindle. In cells that lack IRS2 or express a PLK1-binding deficient mutant, centrosome separation is impaired and mitotic duration is shortened when cells are exposed to mitotic stress, leading to in an increased loss of cell viability. Triple Negative Breast Cancer (TNBC) is characterized by high chromosome instability and high proliferation rates and as a result, TN tumor cells require a strong SAC to respond to mitotic stress. The fact that IRS2 expression is elevated in TNBC and that it contributes to the regulation of the SAC and tumor cell viability in response to mitotic stress identifies a potential IRS2-specific vulnerability that could be exploited therapeutically in this breast cancer subtype. We are continuing to study the mechanism of IRS2 function in mitosis and the consequences of its loss on tumor response to chemotherapeutic drugs that induce mitotic stress.