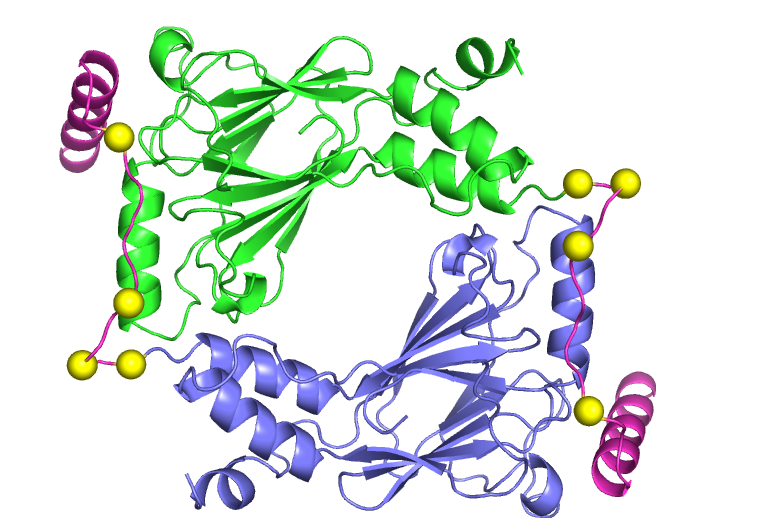
Structural regulation of interferon regulatory factors (IRFs) in the innate immune response
IRF family members play important roles in innate immunity, inflammation and apoptosis. Their clinical importance is evidenced by a strong link between IRF-5 and autoimmune diseases, particularly lupus. Our goal is to obtain a structural understanding of activation that will permit insight into approaches for inactivation that could inform development of future therapies in autoimmune disease. The first step in IRF activation is triggered by phosphorylation of Ser/Thr residues in a C-terminal autoinhibitory region. Phosphylation stimulates dimerization, translocation into the nucleus and assembly with the coactivator CBP/p300 and other proteins to stimulate transcription of type I interferons and other target genes. In collaboration with Dr. Celia Schiffer (BMP) and Dr. Kate Fitzgerald (Medicine), we are continuing work on the structural basis for activation of IRFs that was pioneered by our extraordinary colleague, Dr. Kai Lin, prior his tragic death. Our crystal structure of dimeric pseudophosphorylated IRF-5 (see figure), in comparison with structures of monomeric IRF-3 determined by Dr. Lin, has revealed how phosphorylation triggers a striking conformational rearrangement of the C-terminal region converting it from an autoinhibitory to a dimerization role. Inhibitors of this IRF5 activation have potential as lead compounds in autoimmune disease.