Age-related Macular Degeneration
|
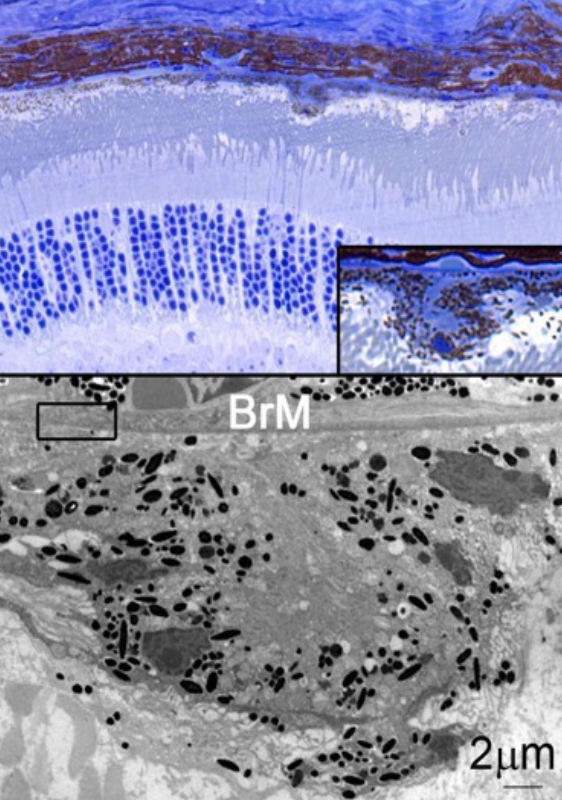
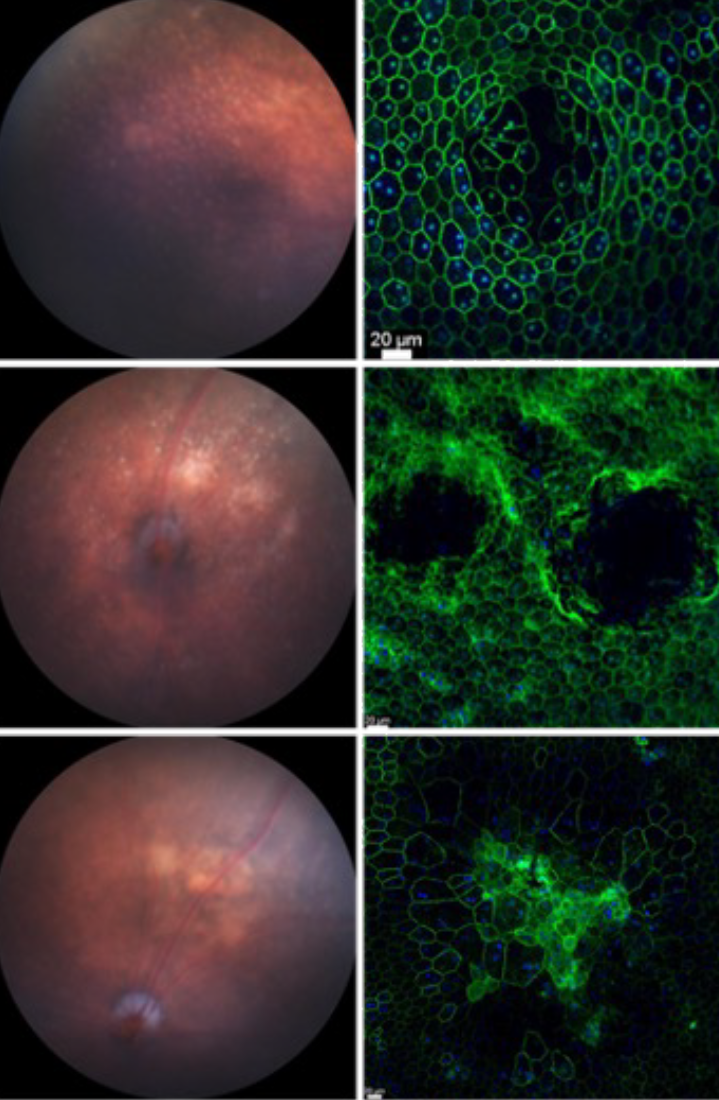
Age-related macular degeneration (AMD) is one of the leading causes for blindness in the industrialized world1. The disease is caused by a multitude of environmental and genetic factors with age being the biggest risk factor2-4. AMD is characterized by the formation of drusen, which are lipid rich deposits that can vary in size, type and location1. Drusen on the basal side of the retinal-pigmented epithelium can be classified into hard or soft drusen. While hard drusen up to a certain size are part of the normal aging process and are also found in the peripheral retina5, soft drusen on the other hand are a hallmark of the disease pathology and are generally located in the macula area, the center of high acuity vision in humans. Drusen in the subretinal space are referred to as subretinal drusenoids, which have been shown to increase the risk of disease progression6,7.
As the disease advances and drusen become larger, loss of visual function occurs due to retinal-pigmented epithelium and photoreceptor atrophy (dry AMD). Extended patches of retinal-pigmented epithelium and photoreceptor loss are usually referred to as geography atrophy8. In some cases, the progression of the disease can take an alternative path resulting in neovascular invasion of the choriocapillaris across the Bruch’s membrane and retinal-pigmented epithelium into the photoreceptor layer1. Leakage from these newly formed vessels can cause subretinal hemorrhage accelerating retinal-pigmented epithelium and photoreceptor loss. This stage of the disease is commonly referred to as wet AMD. It affects roughly 15% of all AMD patients’ age 80 and older and if left untreated, it can result in complete central vision loss4. Today, wet AMD is being successfully treated with anti-angiogenic factors (anti Vascular Endothelial Growth Factor: Lucentis)2,9. In contrast, treatments for dry AMD and geographic atrophy remain exploratory2. The only treatments currently available to reduce the risk of developing advanced AMD are a combination of antioxidants and zinc10, and high dose supplementation with omega 3 fatty acids11.
|
How drusen develop and cause photoreceptor loss remains still unclear. It is has been proposed that with age, accumulated oxidative stress in retinal-pigmented epithelium cells affects their ability to fully digest the phagocytosed photoreceptor outer segments12-15. This would result in intracellular accumulation of only partially digested material that would then be secreted towards the basal side of the cell. Given the workload of an retinal-pigmented epithelium cell, which is to provide photoreceptors with nutrients and oxygen, recycle the visual chromophore and digest 10% of roughly 30 photoreceptor outer segments daily16-18, it is reasonable to assume that with age retinal-pigmented epithelium cells becomes less efficient. Because photoreceptor outer segments are rich in lipids19-21, cholesterol22 and proteins23, inefficiently digested outer segment could also perturb cholesterol and lipid homeostasis within the retinal-pigmented epithelium24. Such perturbations should vary regionally because of the different distribution of rods and cones and their different lipid and cholesterol contents in their outer segments. In fact, newer models seem to attribute the retinal-pigmented epithelium problem to a lipid imbalance in the retinal-pigmented epithelium itself discounting the role of photoreceptor outer segment digestion yet accounting for the high and differential lipid requirements of rods and cones22,25. We think the problem is a combination of both, where age-related metabolic changes in photoreceptors cause a lipid imbalance in the retinal-pigmented epithelium because the retinal-pigmented epithelium digests photoreceptor on a daily base. Based on that we have generated a mouse model where we perturb photoreceptor metabolism alone. Our model develops classical features of AMD including geographic atrophy. Read more in our newly published research.
Bibliography
1. Miller, J.W. Age-related macular degeneration revisited--piecing the puzzle: the LXIX Edward Jackson memorial lecture. Am J Ophthalmol 155, 1-35 e13 (2013).
2. Yonekawa, Y., Miller, J.W. & Kim, I.K. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J Clin Med 4, 343-359 (2015).
3. Fritsche, L.G., et al. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet 15, 151-171 (2014).
4. Jager, R.D., Mieler, W.F. & Miller, J.W. Age-related macular degeneration. N Engl J Med 358, 2606-2617 (2008).
5. Ardeljan, D. & Chan, C.C. Aging is not a disease: distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 37, 68-89 (2013).
6. Huisingh, C., et al. The Association Between Subretinal Drusenoid Deposits in Older Adults in Normal Macular Health and Incident Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci 57, 739-745 (2016).
7. Zarubina, A.V., et al. Prevalence of Subretinal Drusenoid Deposits in Older Persons with and without Age-Related Macular Degeneration, by Multimodal Imaging. Ophthalmology 123, 1090-1100 (2016).
8. Danis, R.P., Lavine, J.A. & Domalpally, A. Geographic atrophy in patients with advanced dry age-related macular degeneration: current challenges and future prospects. Clin Ophthalmol 9, 2159-2174 (2015).
9. Kendall, R.L. & Thomas, K.A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 90, 10705-10709 (1993).
10. McCusker, M.M., Durrani, K., Payette, M.J. & Suchecki, J. An eye on nutrition: The role of vitamins, essential fatty acids, and antioxidants in age-related macular degeneration, dry eye syndrome, and cataract. Clin Dermatol 34, 276-285 (2016).
11. Georgiou, T. & Prokopiou, E. The New Era of Omega-3 Fatty Acids Supplementation: Therapeutic Effects on Dry Age-Related Macular Degeneration. J Stem Cells 10, 205-215 (2015).
12. Hanus, J., Anderson, C. & Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res Rev 24, 286-298 (2015).
13. Beatty, S., Koh, H., Phil, M., Henson, D. & Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol 45, 115-134 (2000).
14. Grindle, C.F. & Marshall, J. Ageing changes in Bruch's membrane and their functional implications. Trans Ophthalmol Soc U K 98, 172-175 (1978).
15. Young, R.W. Pathophysiology of age-related macular degeneration. Surv Ophthalmol 31, 291-306 (1987).
16. Snodderly, D.M., Sandstrom, M.M., Leung, I.Y., Zucker, C.L. & Neuringer, M. Retinal pigment epithelial cell distribution in central retina of rhesus monkeys. Invest Ophthalmol Vis Sci 43, 2815-2818 (2002).
17. Young, R.W. The renewal of photoreceptor cell outer segments. J Cell Biol 33, 61-72 (1967).
18. Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J Cell Biol 49, 303-318 (1971).
19. Bownds, D., Gordon-Walker, A., Gaide-Huguenin, A.C. & Robinson, W. Characterization and analysis of frog photoreceptor membranes. J Gen Physiol 58, 225-237 (1971).
20. Scott, B.L., Racz, E., Lolley, R.N. & Bazan, N.G. Developing rod photoreceptors from normal and mutant Rd mouse retinas: altered fatty acid composition early in development of the mutant. J Neurosci Res 20, 202-211 (1988).
21. Whikehart, D.R. Biochemistry of the Eye, 2nd Edition, (2003).
22. Pikuleva, I.A. & Curcio, C.A. Cholesterol in the retina: the best is yet to come. Prog Retin Eye Res 41, 64-89 (2014).
23. Wang, L., et al. Abundant lipid and protein components of drusen. PLoS One 5, e10329 (2010).
24. Curcio, C.A., et al. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina 33, 265-276 (2013).
25. Curcio, C.A., Johnson, M., Huang, J.D. & Rudolf, M. Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins. Prog Retin Eye Res 28, 393-422 (2009).
