Control of Membrane Localization
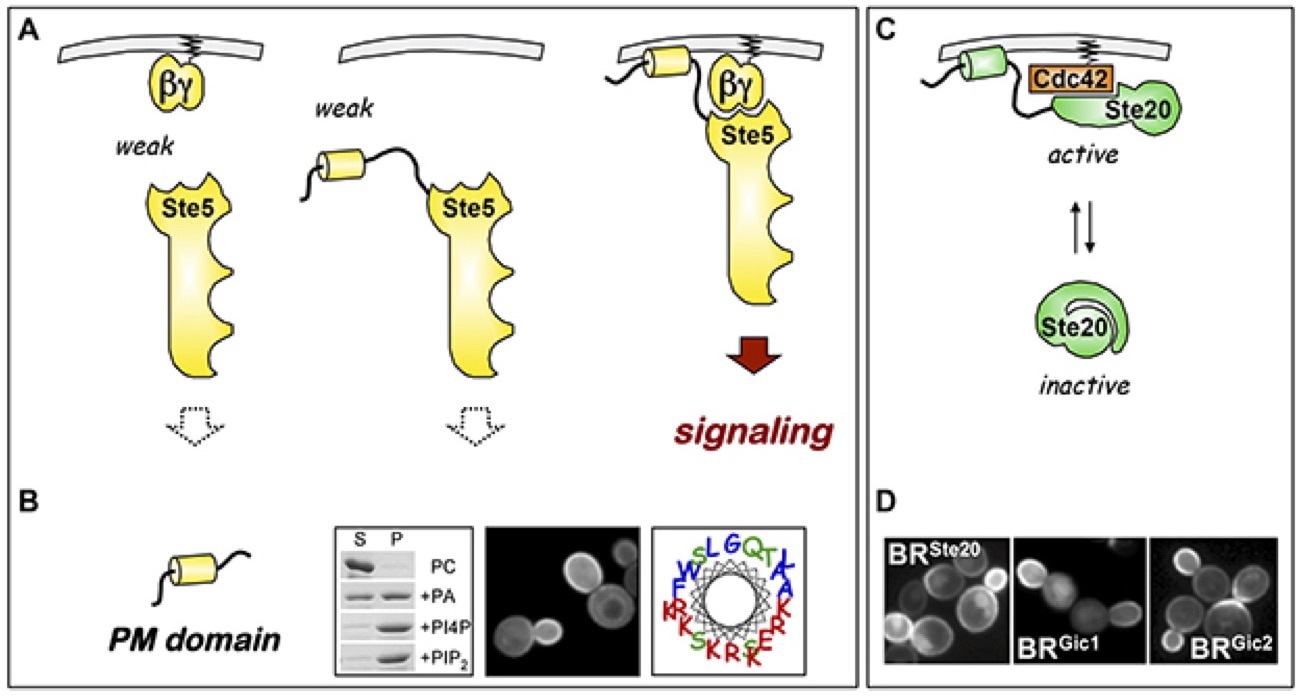
The protein Ste5 plays a critical role in pheromone signaling. It is called a "scaffold" protein for its ability to bind multiple kinases of the MAP kinase cascade and promote their interaction. An important early step in pheromone response is the recruitment of Ste5 to the plasma membrane, which is mediated by the G protein beta-gamma dimer (Gβγ). Yet, this interaction is not sufficient. Instead, Ste5 recruitment requires a synergistic effect of two weak interactions: a protein-protein interaction and a protein-membrane interaction. The membrane interaction is mediated by a sequence called the PM domain, which is a putative amphipathic alpha helix that binds acidic phospholipid membranes in vivo and in vitro. We also found similar domains, and a similar synergistic localization, in other signaling proteins. Currently, we continue to explore new sequences, and investigate the potential benefits of synergistic binding; possibilities include greater evolutionary plasticity, increased spatial and temporal dynamics, and expanded opportunities for signal integration.