Structure/function of vertebrate myosin filaments
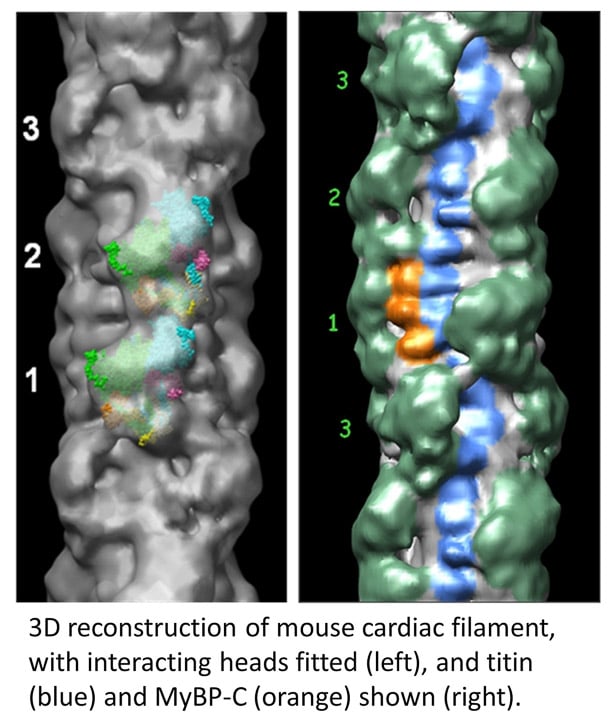
The skeletal and cardiac muscles of vertebrates are important because of their relevance to human physiology and disease. However, vertebrate filaments are more challenging to study than invertebrates, as they are less stable and more complex (with proteins in addition to myosin, e.g. titin, MyBP-C). Using single particle techniques and negative staining we determined the organization of the myosin heads, titin, and MyBP-C (Zoghbi et al., 2008) in mouse cardiac filaments. The myosin heads have a similar interacting-head structure to that in invertebrates (left figure). The reconstruction also shows the layout of the giant protein, titin (blue beads in right figure), running along the filament surface , and the organization of MyBP-C’s C-terminal domains (orange), binding to the filament backbone. We are currently pursuing high resolution reconstructions of vertebrate filaments (both skeletal and cardiac) by cryo-EM. We are also determining the impact of myosin-targeted therapeutic drugs (mavacamten, omecamtiv mecarbil) on filament structure.