Myosin Filament Structure - Invertebrates
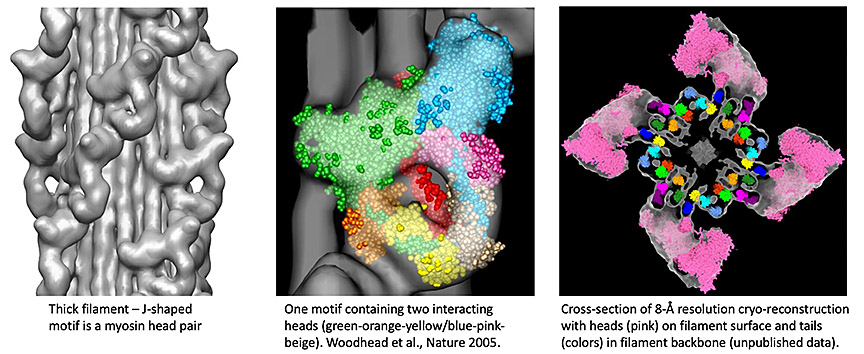
Myosin filaments are polymers of myosin II. For decades there was little understanding of how filaments were constructed nor how myosin motors functioned. We have used conventional cryo-EM to preserve native filament structure, and single particle techniques to carry out 3D reconstruction of myosin filaments from tarantula, a model system for filament structure (Woodhead et al., Nature 2005). Our results show that myosin’s two heads interact with each other in relaxed muscle. This blocks sites needed for actin interaction and ATP hydrolysis, switching filament activity off. We recently showed that this interacting-heads motif (IHM) is common to thick filaments across the evolutionary tree. It arose early in evolution, is fundamental to muscle function, and represented a paradigm-shift in our understanding of muscle structure/function. Our current research focuses on the atomic-level interactions that underlie this inhibitory activity. We are using the state-of-the-art cryo-EM instrumentation at the medical school to obtain atomic detail.