Kidney development and disease
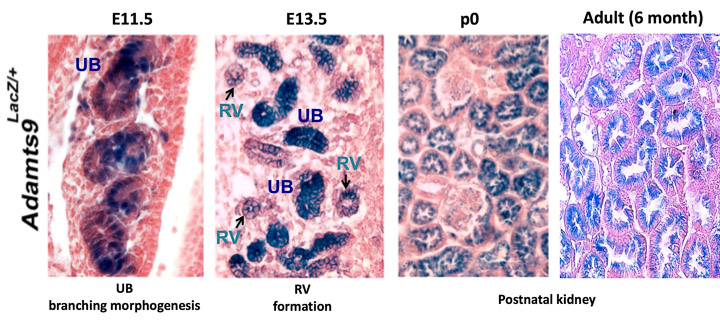
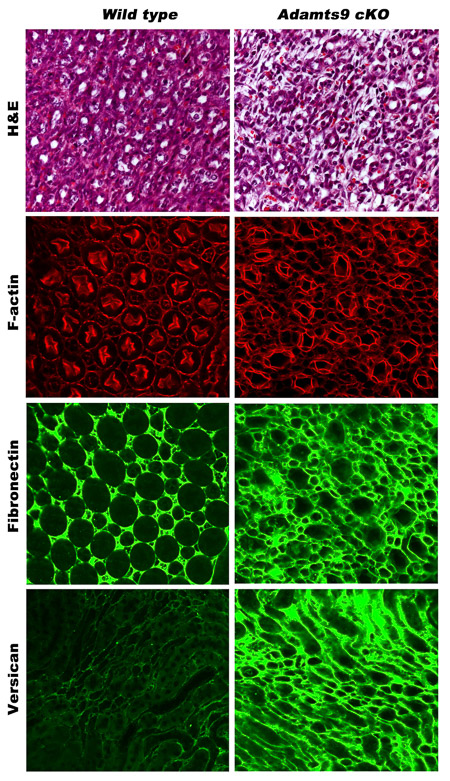 Primary cilia are crucial for normal kidney development and function. Failure to sense urine flow in kidney tubules leads to severe cystic kidney diseases such as PKD (polycystic kidney disease) and nephronophthisis (NPHP). Furthermore, extracellular matrix (ECM) homeostasis is also crucial for normal kidney function and accumulation of ECM leads to nephron death and chronic kidney disease. ADAMTS9, a well characterized ECM protease lies in the crossroads of both these crucial processes. ADAMTS9 mutations in humans cause NPHP. Adamts9 is highly expressed in the mouse kidney from the earliest stages of kidney development (image-1). However, the role of Adamts9 in mouse kidney development has not been elucidated to date. Preliminary studies depleting Adamts9 in conditional knock out mouse models show ECM accumulation and disrupted kidney tubules (image-2). In this project, we will investigate the ADAMTS9 kidney degradome in mice mouse and in iPSC-derived normal human kidney organoids and NPHP models. We will investigate the effects of Adamts9 loss in mouse kidneys and characterize altered ECM dynamics, ciliogenesis defects, altered cell signaling, altered proteomes and the histopathology of the knockout kidneys. These studies will establish crucial models to understand and characterize ADAMTS9 function in human kidney development and disease onset.
Primary cilia are crucial for normal kidney development and function. Failure to sense urine flow in kidney tubules leads to severe cystic kidney diseases such as PKD (polycystic kidney disease) and nephronophthisis (NPHP). Furthermore, extracellular matrix (ECM) homeostasis is also crucial for normal kidney function and accumulation of ECM leads to nephron death and chronic kidney disease. ADAMTS9, a well characterized ECM protease lies in the crossroads of both these crucial processes. ADAMTS9 mutations in humans cause NPHP. Adamts9 is highly expressed in the mouse kidney from the earliest stages of kidney development (image-1). However, the role of Adamts9 in mouse kidney development has not been elucidated to date. Preliminary studies depleting Adamts9 in conditional knock out mouse models show ECM accumulation and disrupted kidney tubules (image-2). In this project, we will investigate the ADAMTS9 kidney degradome in mice mouse and in iPSC-derived normal human kidney organoids and NPHP models. We will investigate the effects of Adamts9 loss in mouse kidneys and characterize altered ECM dynamics, ciliogenesis defects, altered cell signaling, altered proteomes and the histopathology of the knockout kidneys. These studies will establish crucial models to understand and characterize ADAMTS9 function in human kidney development and disease onset.
Versican, a large chondroitin sulfate proteoglycan, is the best characterized ECM substrate of ADAMTS9. Increased versican levels are highly detrimental for kidney function, nephron health and survival. Therefore, in addition to ADAMTS9 function in the mammalian kidney, we will also investigate the effects of increased versican utilizing a novel versican mutant mouse (VcanAA) where the ADAMTS cleavage site has been mutated uncleavable.