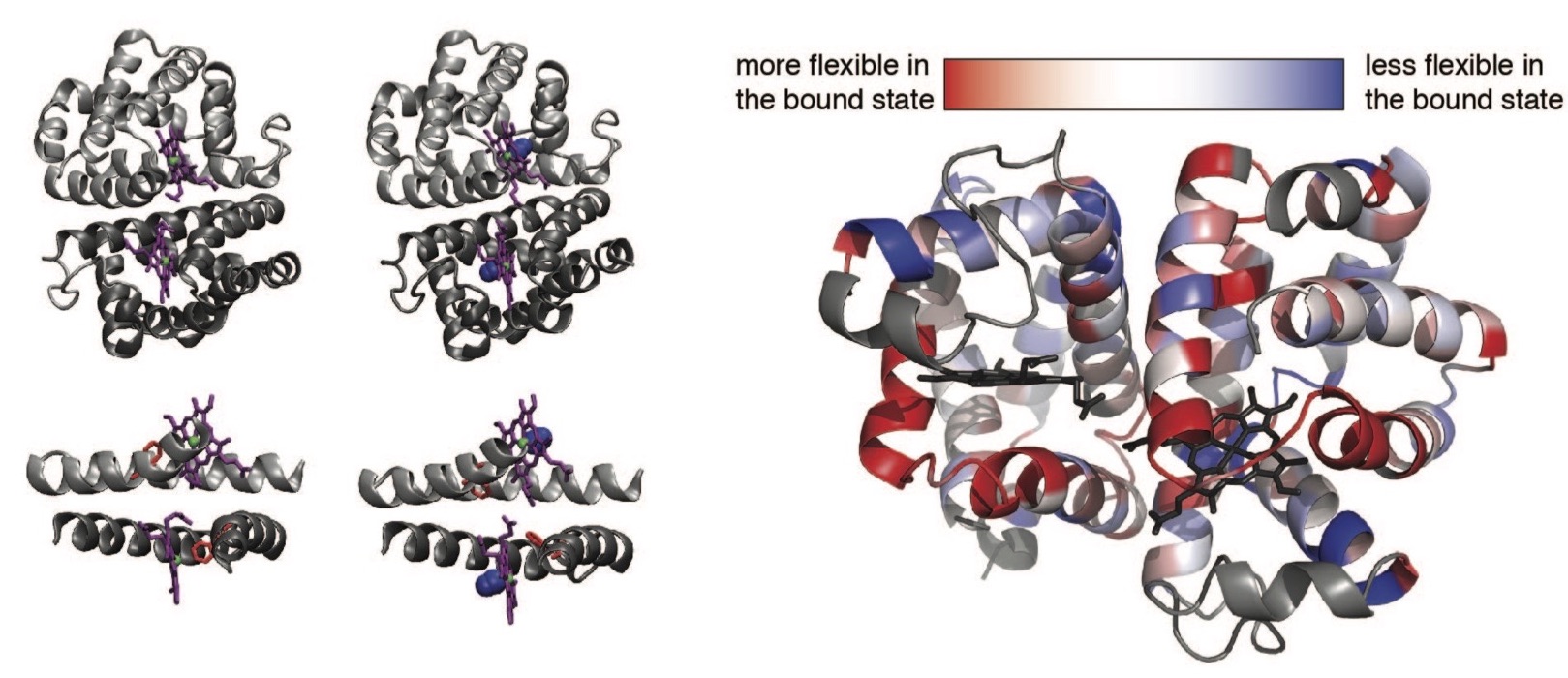
The Allosteric Mechanism of Scapharca Dimeric Hemoglobin (HbI)
Protein dynamics and allostery:
Allosteric regulation is an essential function of many proteins that control a variety of different processes such as catalysis, signal transduction, and gene regulation. Structural rearrangements have historically been considered the main means of communication between different parts of a protein. Recent studies have highlighted the importance, however, of changes in protein flexibility as an effective way to mediate allosteric communication across a protein. We are characterizing the contributions of dynamics to allosteric function using a homodimeric hemoglobin from Scapharca inaequivalvis (HbI).
Interface flexibility is important for the cooperative mechanism of HbI:
We have used MD simulations and NMR relaxation techniques to discover that the fast internal motions of HbI contribute to the cooperative binding to carbon monoxide in two ways: 1) by contributing favorably to the free energy of the system and 2) by participating in the cooperative mechanism at the HbI subunit interface. We are interested in investigating the coupling between the change in structure and dynamics of the interfacial helices (E and F) and the interfacial water to characterize its contribution to the cooperative mechanism of HbI.