Our Approach
The prime value of identifying new genes is that it lets us identify the molecular components, pathways and processes that become defective in ALS patients. Targeting such pathways will make it possible to develop new therapeutics for all ALS patients, not just those with a mutation in a specific gene. For instance, our previous discoveries of the ALS genes PFN1, TUBA4A, and NEK1 gave us information suggesting that both genes are involved in the structure and maintenance of the cytoskeleton (see research section). Our latest discovery of KIF5A, another cytoskeleton-related gene, further solidified our belief that the cytoskeleton plays a vital role in the neuronal degeneration observed in all ALS patients. The information gained from our genetic studies may very well lead to a therapeutic treatment for ALS patients.
Over the past few years, my laboratory has focused on identifying potential therapeutics that can alleviate the cytoskeletal defects observed in ALS patients. We led a multi-institutional effort to identify such therapeutics with groups from University of California San Francisco, Brandeis University and other members of UMass Chan Medical School. Our concept is relatively simple. We had shown that if you place a mutant ALS gene into healthy neurons, they die in typically one week. As such, we can test a library of different “compounds” to identify those that prevent the neurons from dying. Such “hits” can then be tested in additional cellular and animal models of ALS. Although our budget was very limited and allowed screening of only ~220 “compounds”, the assay identified one compound that could rescue dying neurons harbouring any of three different mutant ALS genes. We are currently testing this compound in additional cellular and animal models of ALS.
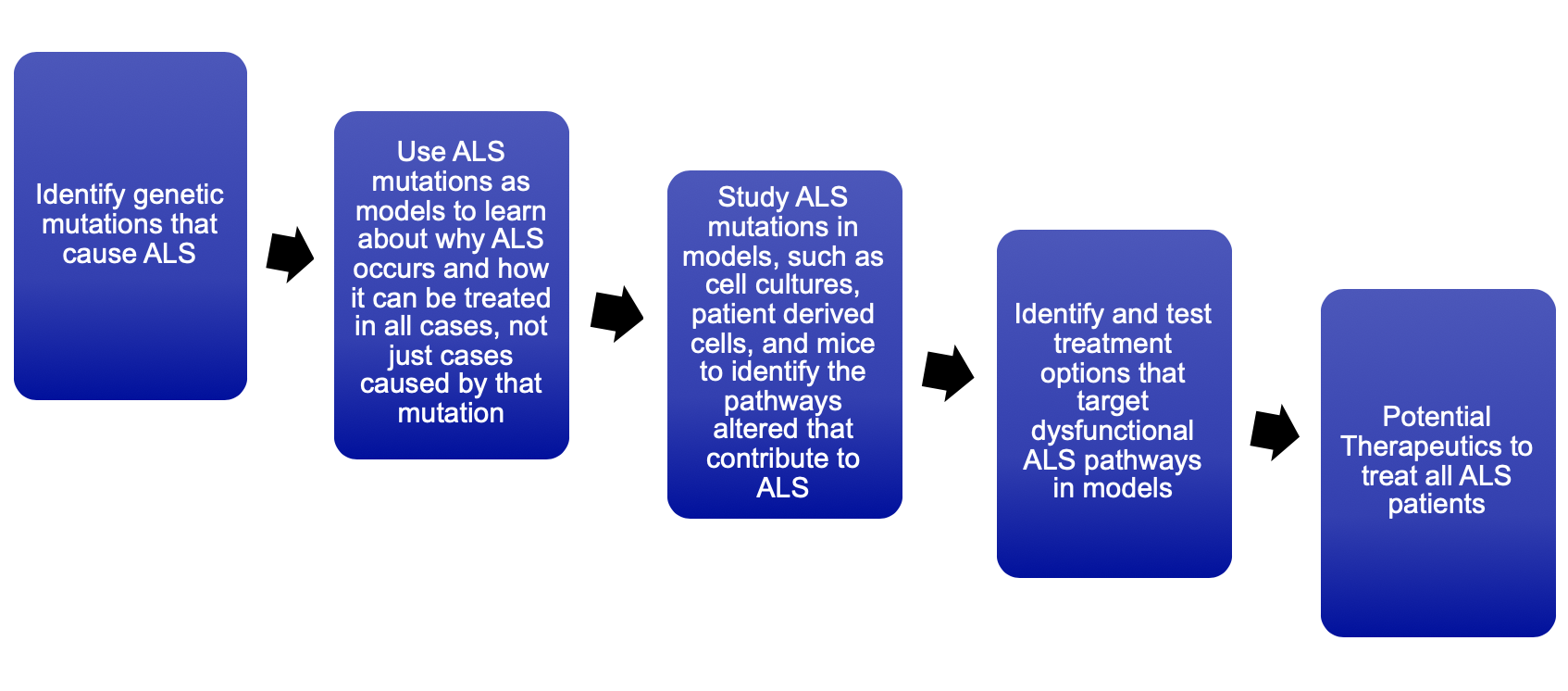
It is truly my belief that through our continued efforts, we can identify one of the first true therapeutics for ALS patients in over 20 years. It is my full intent to expand our ability to screen for compounds that can rescue dying neurons resulting from mutant ALS genes altering the cytoskeleton. We intend to expand our screening capacity beyond a few hundred compounds to tens of thousands. Furthermore, we intend to establish high-throughput approaches to quickly test our hits in numerous cellular and animal models.
Unfortunately, as I am sure you aware, scientific research is expensive. To accomplish these goals and convert our genetic findings into patient therapeutics requires money. We are actively seeking funds through government grants and charitable foundations; however, funds from these sources can be difficult to obtain, require great lengths of time to acquire and are limited in their amounts available.
Due to the challenges with obtaining grants, I am asking for donations to support our work and allow it to continue. Our primary goal is a direct therapeutic for all ALS patients. My track record proves our distinctive capabilities to reach our goal and the strengths of collaborations with all members of the ALS scientific community to expedite this work. With your support and assistance, our lab can continue to drive innovative discovery in new ALS genes and help us identify therapeutics to truly change lives.