Research Areas
Thalamic targeting for deep brain stimulation and focused ultrasound
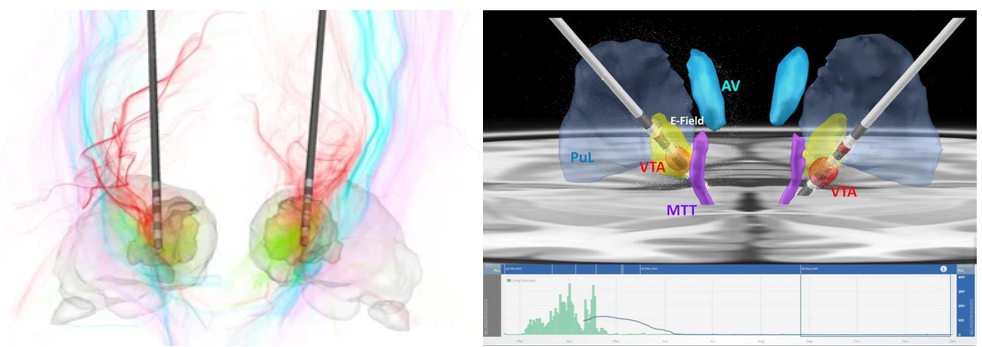
Thalamic nuclei are increasing targeted for deep brain stimulation (DBS) applications and for ablation using focused ultrasound (FUS). The earliest use is targeting the ventral intermediate (VIM) nucleus for treatment of essential tremor using both DBS and FUS. The methods we have developed for visualization and segmentation of thalamic nuclei (see sections below) have been helping neurosurgeons with accurate targeting of these deep brain nuclei. Top left is a recent use of the THOMAS algorithm for DBS of the centrolateral (CL) nucleus for traumatic brain injury reported by Schiff et al. Top right panel shows the use of THOMAS to visualize the targeting of centromedian (CM) nucleus for epilepsy by Ganne et al. The bottom EEG recording shows dramatic reduction in seizures following implantation of DBS electrodes in CM.
Thalamic nuclei atrophy in neurodegenerative disease
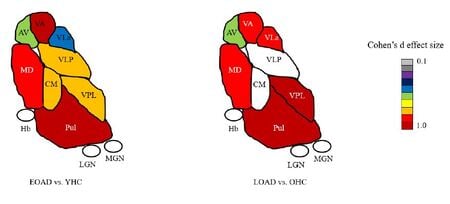
Most neuroimaging studies view the thalamus as a unitary structure, missing out on the specificity of thalamic nuclei and their role in disease. For e.g. Alzheimer's disease researchers have focused almost exclusively on the hippocampus even though the anterior thalamic nuclei is part of the limbic memory circuit (Papez circuit) responsible for episodic memory. We have shown thalamic nuclei atrophy in early MCI that even precedes hippocampal atrophy. This atrophy progressively increases with disease stage. We have recently published differences in early vs. late onset AD in thalamic nuclear atrophy (see image left). Our group has found similar nuclei specific atrophy in the ventral posterolateral nucleus (VPL) in alchohol use disorder, accompanied by concomitant disruption in functional connectivity. We have also demonstrated specific atrophy of thalamic nuclei close to the third ventricle (mediodorsal, pulvinar etc) in multiple sclerosis. We are currently examining thalamic nuclei involvement in familial AD, frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS) and schizophrenia. Please see publications tab for specific papers.
Visualization of thalamic nuclei
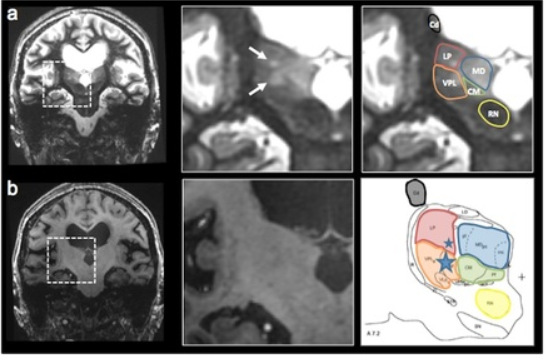
Thalamic nuclei are almost invisible on standard T1 and T2 MRI. We have been pioneering novel methods for visualizing thalamic nuclei. These are critical for many applications like deep brain stimulation (DBS) electrode placement and focused ultrasound ablation of thalamic nuclei for treatment of essential tremor. They are also important to study atrophy of these nuclei in various neurodegenerative and neuro-psychiatric disorders. We have developed and optimized a novel white-matter-nulled (WMn) MRI sequence which enables clearer delineation of thalamic nuclei (top left, top centre) compared to conventional T1 MP-RAGE sequence (bottom left, bottom centre). Manual segmentation of some thalamic nuclei and the Morel atlas are shown in the far right column.
Fast automated segmentation of thalamic nuclei
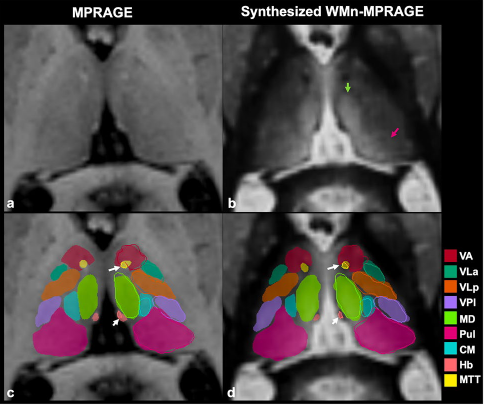
THalamus Optimized Multi Atlas Segmentation (THOMAS) is a state of the art method we have developed for segmentation of WMn MRI data. It uses 20 prior manually segmented data sets for segmentation of new input data. We have also adapted THOMAS to segment the more readily available T1 MRI data, albeit with some reduction in accuracy. THOMAS has also been modified to create an atlas in MNI space which has been incorporated into leaddbs, a very popular open source software for DBS lead placement in neurosurgery.
More recently, we have been exploring sophisticated deep-learning methods to improve accuracy and speed of thalamic nuclear segmentation. One method synthesizes WMn MRI data from standard T1 MRI data and segments it using convolutional neural networks (CNN), opening the door for rapid analysis of databases such as ADNI, OASIS, and HCP. We have also developed a simpler more robust version using polynomial synthesis.
Fast dynamic MRI
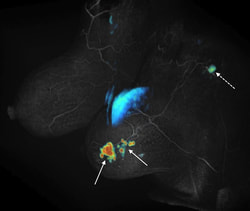 Spatial and temporal resolution are competing requirements in dynamic MRI, often requiring compromises. We are developing methods based on view sharing, compressed sensing and combinations of the two along with novel acquisition schemes to accelerate dynamic contrast enhanced imaging. The main applications are in cancer imaging (breast, prostate, liver) as well as quantification of renal function. One such method called Differential Subsampling with Cartesian Ordering or DISCO is now a product on GE MRI scanners. The picture on the left shows initial slope of contrast enhancement wash-in overlaid on a DISCO post contrast image obtained from a patient with invasive ductal carcinoma (solid arrows) with a likely metastatic axillary node (dotted arrow).
Spatial and temporal resolution are competing requirements in dynamic MRI, often requiring compromises. We are developing methods based on view sharing, compressed sensing and combinations of the two along with novel acquisition schemes to accelerate dynamic contrast enhanced imaging. The main applications are in cancer imaging (breast, prostate, liver) as well as quantification of renal function. One such method called Differential Subsampling with Cartesian Ordering or DISCO is now a product on GE MRI scanners. The picture on the left shows initial slope of contrast enhancement wash-in overlaid on a DISCO post contrast image obtained from a patient with invasive ductal carcinoma (solid arrows) with a likely metastatic axillary node (dotted arrow).
Pharmacokinetic modeling and multi-parametric imaging
Quantitative modeling of contrast uptake in tumors can improve the specificity of MRI (i.e. distinguish benign vs. malignant lesions) but requires good SNR and high temporal resolution, often hard to meet clinically. In a typical MRI exam, images with multiple contrasts (T2, diffusion-weighted, T1) are acquired. The use of shape based metrics may permit extraction of quantitative parameters from poor quality data. Machine learning based techniques can help analyze and characterize multi-parametric data in ways which are not feasible using current qualitative analyses.