Mechanisms of Translation Regulation
Non-canonical Translation Initiation
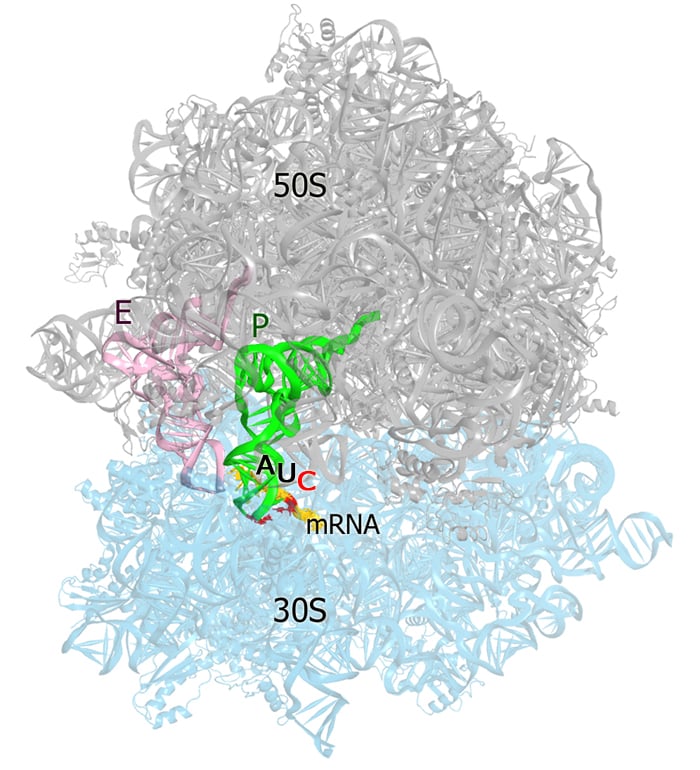
Translation of cellular mRNAs normally initiates on an AUG start codon, but non-AUG initiation can occur to regulate translation of a subset of mRNAs, for example by establishing an alternative open reading frame. To gain isight into the mechanism of non-AUG initiation, we have solved a crystal structure of the 70S initiation complex containing an AUC codon in the ribosomal P (peptidyl-tRNA) site. The AUC codon results in a cytosine-cytosine mismatch at third position of the RNA helix formed by the mRNA codon and initiator-tRNA anticodon. A C-C mismatch usually confers substantial destabilization to nucleic-acid structure, and disrupts helices and tertiary interactions. Remarkably, the ribosome stabilizes the mismatched codon-anticodon helix, arranging the cytosine-cytosine pair into a nearly planar conformation, resembling a Watson-Crick base pair formed by complementary bases (e.g. G-C). Translation-competent conformations of the tRNA, mRNA, and decoding center demonstrate how the ribosomal P site helps to establish and maintain the open reading frame. Svidritskiy E, Korostelev AA. "Ribosome Structure Reveals Preservation of Active Sites in the Presence of a P-Site Wobble Mismatch" . Structure. 2015. The large (50S) subunit of Thermus thermophilus 70S ribosome is shown in gray, the small subunit (30S) is cyan, the cytosine-cytosine mismatch is red.