Mechanisms of Translation Regulation
Understanding Bacterial Pathogenesis - ribosome as a stress sensor
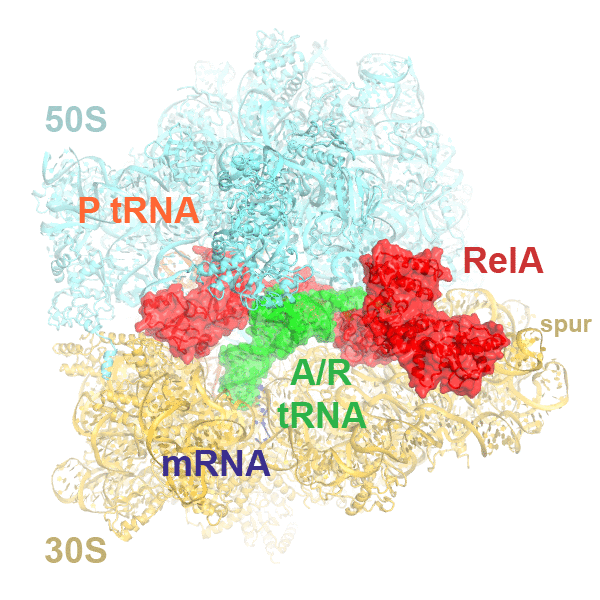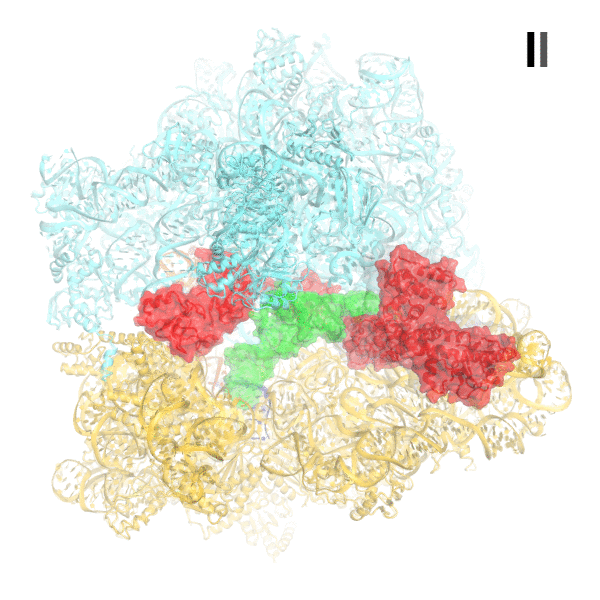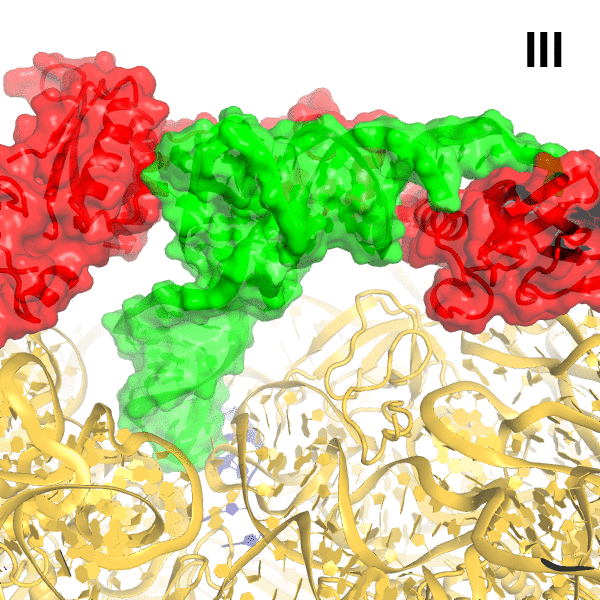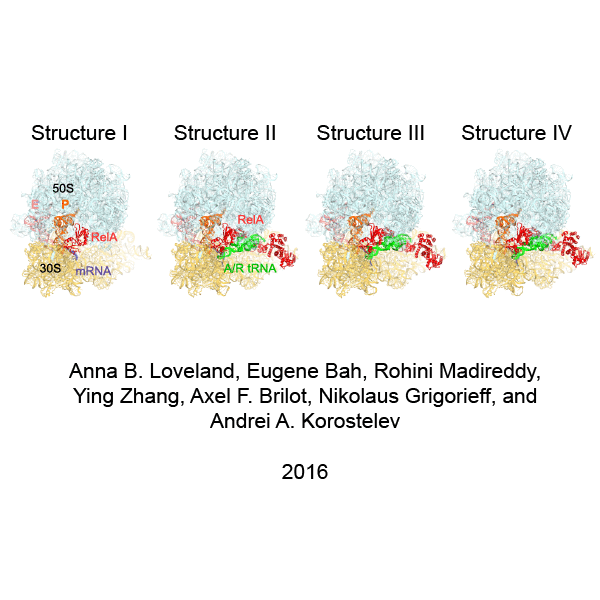
In bacteria, the stringent response is a key driver of virulence and antibiotic resistance. In response to cellular stresses, such as nutrient deprivation, RelA or its homologs (RSH proteins) initiate the stringent response by synthesizing small-molecule 'alarmones' collectively called (p)ppGpp (guanosine pentaphosphate and guanosine tetraphosphate). Accumulation of (p)ppGpp allows cells to adapt to stress conditions. Understanding the molecular mechanism of stringent response initiation - via RelA activation - may guide the development of new antibacterial therapeutics. We visualized several cryo-EM structures of RelA on the bacterial 70S ribosome, resolving distinct conformational states in a single sample. They show how deacylated tRNA - which accumulates when stressed bacteria are deprived of amino acids - orders RelA to turn the stringent response on. The dynamics of the tRNA (A/R tRNA) shows how the ribosome selects for the correct (cognate) tRNA by rearrangements in the small (30S) subunit (Loveland et al. "Ribosome•RelA structures reveal the mechanism of stringent response activation". eLife. 2016).
Click on the image above to see an animation showing rearrangements among RelA-bound ribosome structures and tRNA accommodation in the A site.