Discovery and mechanisms of drug action
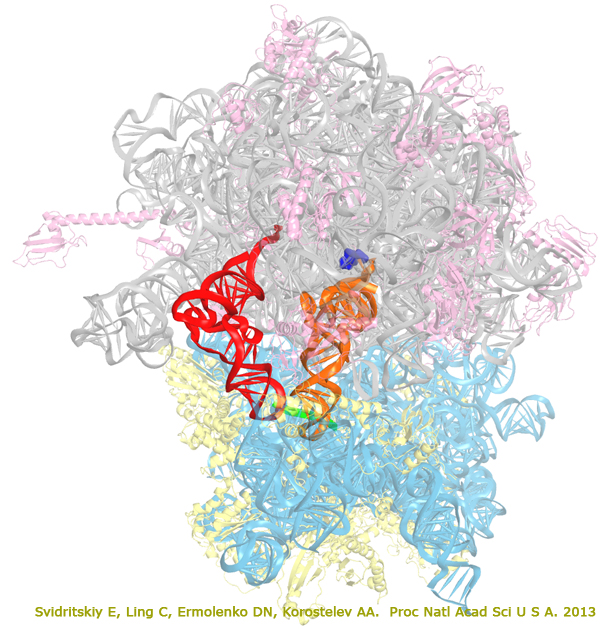 The antibiotic blasticidin S (BlaS) is a potent inhibitor of protein synthesis in bacteria and eukaryotes. To understand its mechanism of action, we have determined a 3.4-A crystal structure of BlaS bound to a 70S*tRNA ribosome complex and performed biochemical experiments. The crystal structure of the antibiotic-ribosome complex reveals that BlaS (blue) binds to the peptidyl-transferase center of the ribosome. The antibiotic impedes protein synthesis through a unique mechanism, by bending the 3' terminus of the P-site tRNA (orange) toward the A site of the large ribosomal subunit. Biochemical experiments demonstrate that stabilization of the deformed conformation of the P-site tRNA by BlaS strongly inhibits peptidyl-tRNA hydrolysis by release factors and, to a lesser extent, peptide bond formation. Svidritskiy et al. "Blasticidin S inhibits translation by trapping deformed tRNA on the ribosome". Proc Natl Acad Sci U S A. 2013. 110(30): 12283–12288. The large (50S) subunit is shown in grey (23S RNA) and pink (proteins), the small (30S) subunit is cyan (16S RNA) and yellow (proteins), messenger RNA is green, P-site tRNA is orange, E-site tRNA is red, blasticidin S is blue.
The antibiotic blasticidin S (BlaS) is a potent inhibitor of protein synthesis in bacteria and eukaryotes. To understand its mechanism of action, we have determined a 3.4-A crystal structure of BlaS bound to a 70S*tRNA ribosome complex and performed biochemical experiments. The crystal structure of the antibiotic-ribosome complex reveals that BlaS (blue) binds to the peptidyl-transferase center of the ribosome. The antibiotic impedes protein synthesis through a unique mechanism, by bending the 3' terminus of the P-site tRNA (orange) toward the A site of the large ribosomal subunit. Biochemical experiments demonstrate that stabilization of the deformed conformation of the P-site tRNA by BlaS strongly inhibits peptidyl-tRNA hydrolysis by release factors and, to a lesser extent, peptide bond formation. Svidritskiy et al. "Blasticidin S inhibits translation by trapping deformed tRNA on the ribosome". Proc Natl Acad Sci U S A. 2013. 110(30): 12283–12288. The large (50S) subunit is shown in grey (23S RNA) and pink (proteins), the small (30S) subunit is cyan (16S RNA) and yellow (proteins), messenger RNA is green, P-site tRNA is orange, E-site tRNA is red, blasticidin S is blue.
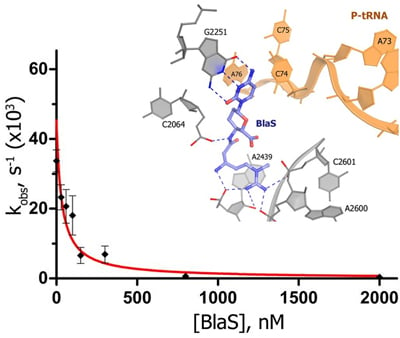
Blasticidin S inhibits RF1-mediated peptide release by binding to the peptidyl-transferase center (Figure shown on the right). Identification of termination inhibitors may lead to development of therapeutics that suppress premature termination codons associated with human diseases, such as cystic fibrosis and Duchenne muscular dystrophy.