Mechanisms of Translation Regulation
Accuracy of Gene Expression
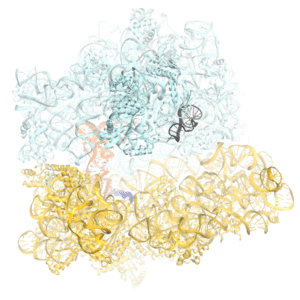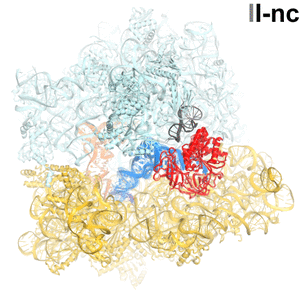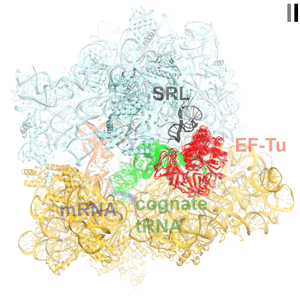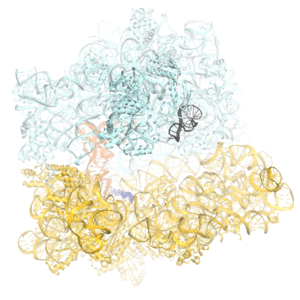
Faithful translation of genetic information into protein relies on accurate decoding of messenger RNA (mRNA). Ribosomes decode mRNA codons by selecting cognate (correct) aminoacyl-tRNAs delivered by elongation factor EF-Tu. Using cryo-EM, we resolved structural ensembles of ribosomes with cognate or near-cognate aminoacyl-tRNAs (Loveland et al, "Ensemble cryo-EM elucidates the mechanism of translation fidelity". Nature. 2017). This work revealed long-sought structural differences between the pre-accommodation of cognate (I, II and III) and near-cognate (I-nc, II-nc, III-nc) tRNAs that elucidate the mechanism of accurate decoding. Both cognate and near-cognate tRNA anticodons explore the decoding center of an open 30S subunit, while inactive EF-Tu is separated from the 50S subunit. A transient conformation of decoding-center nucleotide G530 stabilizes the cognate codon–anticodon helix, initiating step-wise ‘latching’ of the decoding center. The resulting closure of the 30S subunitdomain docks EF-Tu at the sarcin–ricin loop (SRL) of the 50S subunit, activating EF-Tu for GTP hydrolysis (state III) and enabling accommodation of the aminoacyl-tRNA. By contrast, near-cognate complexes (states I-nc and II-nc) fail to induce the G530 latch, thus favouring open 30S pre-accommodation intermediates with inactive EF-Tu.
Click on the image above to see a more detailed animation of the decoding mechanism.