Cardiac Potassium Channel Assembly, Trafficking and Function
What are Cardiac Potassium Channels?
Cardiac potassium channels maintain the rhythmicity of the heartbeat by repolarizing cardiomyocytes such that the electrical and contractile machineries stay in sync. The two major voltage-gated potassium currents responsible for repolarization phase in human cardiomyocytes are cardiac IKs and IKr. The Kobertz lab primarily investigates the KCNQ1/KCNE1 potassium channel complex responsible for generating the slowly activating and deactivating cardiac IKs current.
Cardiac Arrhythmias
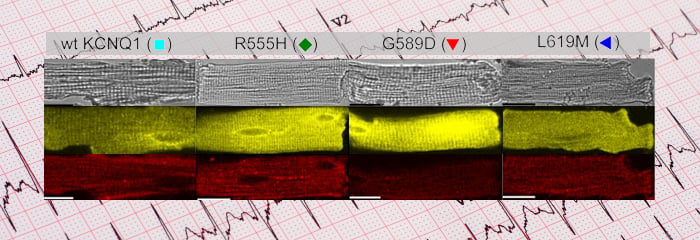
Mutations in potassium conducting subunits (KCNQ1 subunits) or the auxiliary subunits (KCNE1 type I transmembrane peptides) give rise to cardiac arrhythmias. The most common arrhythmia is Long QT syndrome, where mutations in KCNQ1 or KCNE1 give rise to an elongated QT interval, which leaves patients at risk for torsade de pointes. Some patients have congenital deafness due to the KCNQ1/KCNE1 complexes’ key role in supplying the endolymph with potassium. We discovered that N-glycosylation of KCNE1 is vital for the proper trafficking of the complex to the plasma membrane. In addition, we have collaborated with the Colecraft Laboratory at Columbia Medical School to examine the trafficking of mutant KCNQ1/KCNE1 complexes in cardiomyocytes.
Current Cardiomyocyte Research:
Currently, we are determining how mutations in the KCNE1 subunit alter trafficking and cell surface expression. We are also utilizing our panel of KCNE1 glycosylation mutants to investigate co- and post-translational N-glycosylation and O-glycosylation in ventricular cardiomyocytes. The end goal is to understand how the potassium conductance over the entire landscape of cardiomyocyte changes due to inherited mutations and drugs that affect the rhythmicity of the human heart beat. To do this, we are developing a novel technology that enables the fluorescent imaging of extracelluar fluxes