Epigenetic Regulation of Tumor Suppressor Functions
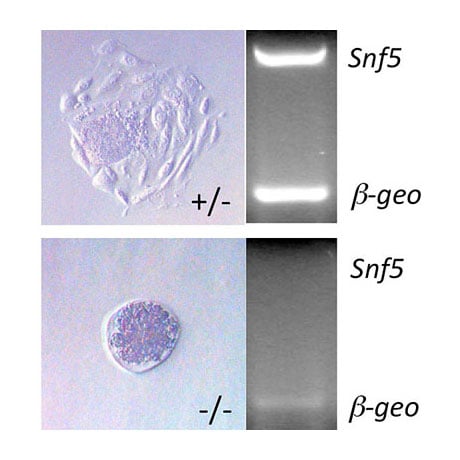
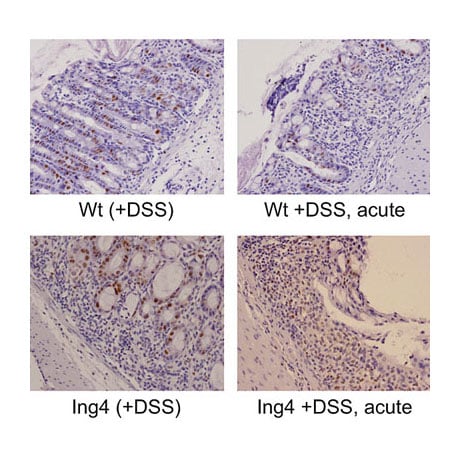
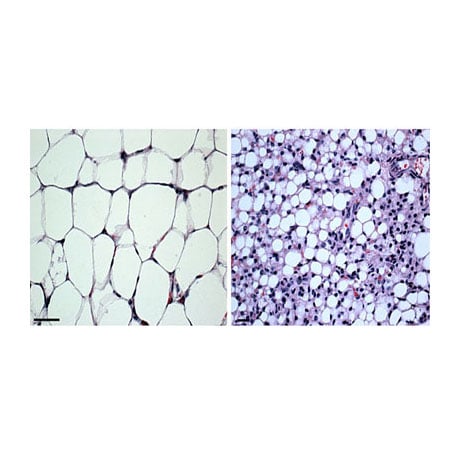
Our lab has generated various other mouse models to explore epigenetic regulation of mouse development and tumorigenesis. We have documented a role of SWI/SNF chromatin remodeling in tumor suppression by deleting the Snf5 gene in mice, and revealed a functional interaction between the Snf5 and Rb tumor suppressors in cell growth and pituitary cancer. In addition, we have also recently examined the role of ING chromatin modifiers in cancer. Our studies have determined that Ing1, a member of the mSin3/HDAC complex, suppresses spontaneous follicular B-cell tumors and collaborates with p53 to prevent diffuse large B cell lymphomas. In contrast, mice deleted for Ing4 (a subunit of the HB01 HAT complex) fail to form tumors, but display defects in innate immunity due to altered NF-kB signaling. Thus ING proteins can function as tumor suppressors and/or alter inflammation in mice. Analysis of the link between inflammation and cancer in these models is ongoing. Lastly, we have also generated Dicer-conditional mice to explore the role of miRNA in the epigenetic regulation of cell growth. Loss of Dicer and miRNA biogenesis activates the p19ARF and p53 tumor suppressors and induces senescence in primary cells during development. Although ablation of Dicer can inhibit the differentiation of various cell types, deletion of Dicer in skin epithelium does not inhibit skin formation, but results in loss of fur. Interestingly, co-deletion of p53 in skin can delay the loss of fur in this model, and mice co-deleted for both p53 and Dicer develop aggressive squamous cell carcinomas. These ongoing studies indicate that miRNAs function to prevent the accumulation of DNA damage in skin epithelium and to suppress skin cancer in mice. The role of miRNAs in activating p53 functions in skin epithelium and identification of the individual miRNA molecules involved in regulating skin cancer is presently being explored.