Adipocytes
Adipocytes are the major energy storage sites in the body, and they also have critical endocrine functions. Therefore, understanding the development and function of adipocytes - particularly in light of the obesity pandemic - is essential to understanding metabolic homeostasis. There are two general classes of adipocytes; white adipocytes - which store energy as a single large lipid droplet and have important endocrine functions, and brown adipocytes - which store energy in multiple small lipid droplets but specifically for use as fuel to generate body heat (i.e. thermogenesis). Heat production by brown adipocytes is made possible by their unique expression of mitochondrial localized uncoupling protein 1 (Ucp1). However, these classifications are oversimplified because some white adipocytes can adopt brown adipocyte characteristics (termed brite or beige adipocytes) and vice versa depending on the temperature and diet. We are interested in understanding the origins of different adipocytes and in defining the signaling and metabolic pathways that control their development, distribution, and function.
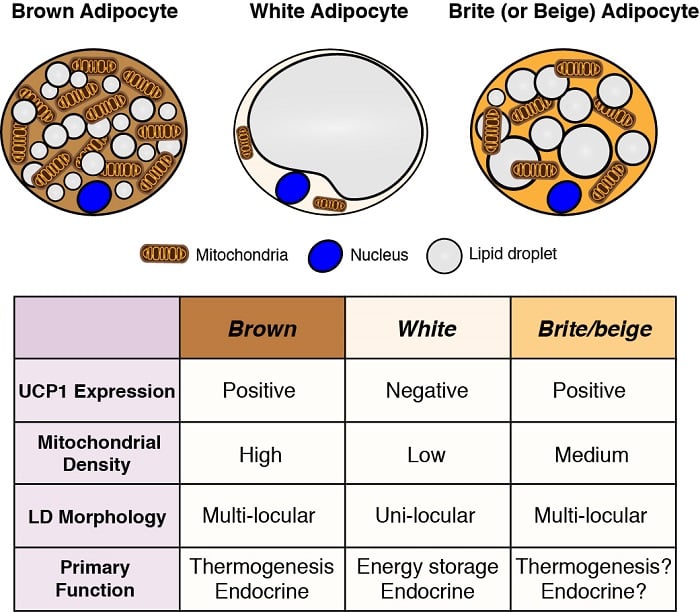
Adipocyte origins
Central to understanding the role of adipose tissue in health and disease is to understand how it grows, and in this respect, one of the least understood areas of adipose tissue biology is the developmental origin of different types of adipocytes. Defining adipocyte origins could help explain human body fat distribution patterns particularly in obese or lipodystrophic individuals, and it may provide clues to metabolic differences observed between some fat depots. Identifying adipocyte precursor cells and the mechanisms regulating their expansion is also critical to understanding and managing healthy adipose tissue function. It may also be possible to engineer the development of “healthy” adipocytes (such as brown or brite/beige adipocytes) from precursors for cell-based therapies aimed essentially at fighting fat with fat. Using a combination of genetics and lineage-tracing strategies we previously mapped the origins of adipocytes residing in different depots. Our results reveal an unexpected level of heterogeneity consistent with adipocytes having multiple developmental origins and supporting a model in which the adipocyte fate likely depends on both extrinsic and intrinsic factors.
Adipocytes have multiple developmental origins
(below left) Anatomical distribution of adipose tissue depots in a mouse. Brown adipose tissue (BAT) and white adipose tissue (WAT) depots are shown. (below right) An example of a lineage tracing experiment during which developmental precursor cells and all of their decendents were indelibly labeled with a fluorescent mark linked to expression of Myf5 (panel a) or Pax3 (panel b). Adipocytes labeled in green (mGFP) originate from a different precursor cell then the adipocytes labeled in red (mTFP).
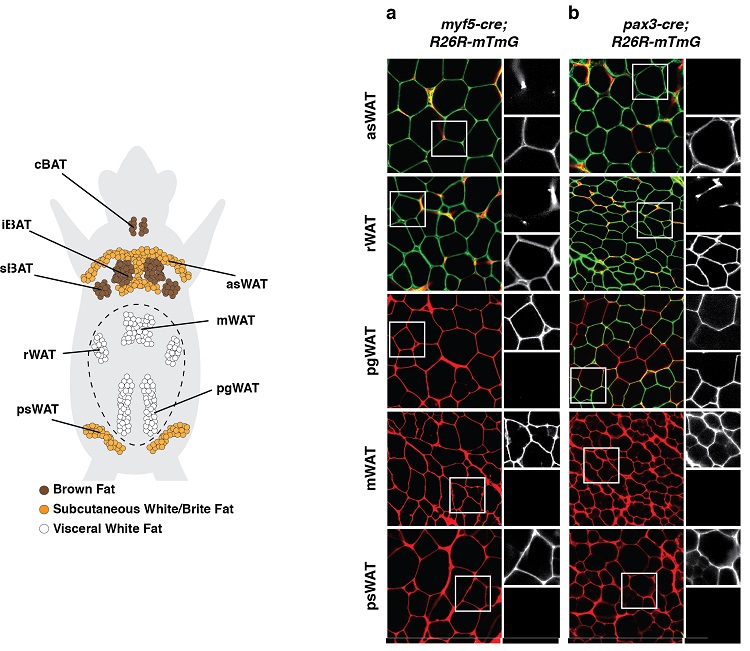
Results such as these suggest adipocytes have multiple developmental origins.
(adapted from Sanchez-Gurmaches & Guertin, Nature Communications 2014; Sanchez-Gurmaches et al., Trends in Cell Biology 2016)
Brown adipocytes
Brown adipocytes are fascinating cells. They only exist in mammals, and their main function is to generate endogenous heat in a process called thermogenesis. This is made possible by their unique expression of a mitochondrial membrane protein called uncoupling protein 1 (UCP1). The energy expending properties of brown fat, and the recent realization that adult humans have brown fat, has made them a target for therapies aimed at fighting overnutrition. Active brown adipocytes also have one of the most intriguing metabolic programs: they take up and consume large amounts of diverse nutrients simultaneously (e.g. glucose, lipids, amino acids) and can simultaneously engage both anabolic and catabolic metabolism. For example, we and others previously showed that cold-stimulated BAT broadly upregulates a genetic program that supports de novo lipid synthesis pathways in addition to fatty acid oxidation pathways [Sanchez-Gurmaches Cell Metabolism 2018]. We would like to understand more about this remarkable and paradoxical metabolism.
Learn more about brown adipose tissue development and metabolism
Anatomical location of brown fat in humans
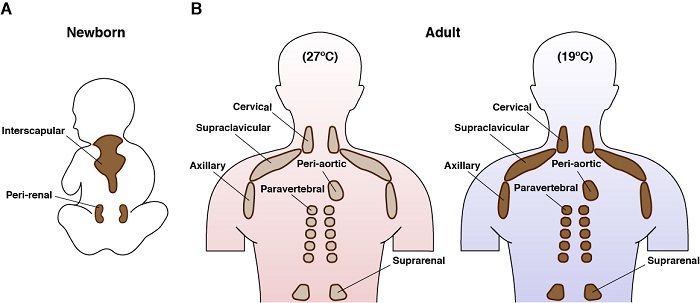
We also use genetic models to investigate how signaling pathways control BAT metabolism. For example, using mice we genetically deleted Rictor - which encodes a unique and essential component of mTORC2 - only in brown adipose tissue. Learn more about mTORC2. Remarkably, these mice are protected from a high fat diet. More specifically, mice lacking BAT Rictor did not accumulate excessive lipids in their liver or visceral adipose tissue depots when eating the high fat diet [Jung et al Molecular Cell 2019]. This is an exciting finding as excess lipid storage in these sites is dangerously unhealthy. Using a combination of genetics, genomics, metabolomics and biochemistry, we are trying to understand why these mice are protected from high fat diet.
White adipocytes
White adipocytes are the most abundant adipocytes in humans. White adipose tissue depots also have a remarkable ability to expand and store energy, and they signal to the brain and other tissues to strongly influence feeding behavior and metabolic homeostasis. However, while white adipocytes are specifically adapted to safely lock up excess nutrients, they have a tipping point (e.g. in obesity) in which their beneficial functions fail, and this strongly promotes the onset of metabolic disease and type 2 diabetes. What defines the tipping point? How do white adipocytes signal to other tissues? How heterogeneous are white adipose tissue depots? Why is having excess visceral fat more detrimental to your health than having excess subcutaneous fat? These are all outstanding questions that we are interested in.
We are also studying mTOR signaling in white adipocytes. mTOR is a major downstream target of insulin signaling, which is the major hormone that adipocytes respond to. Using genetic mouse models, we selectively inhibited either mTORC1 or mTORC2 in all adipocytes. Inhibiting mTORC1 in white adipose tissue causes a lipodystrophy like syndrome associated with insulin resistance and fatty liver disease. Inhibiting mTORC2 in white adipose tissue also causes insulin resistance, but independently of changes in adipose tissue mass. In the latter model, mTORC2 appears to regulate an adipocyte-derived signal that communicates with the liver to control hepatic glucose production. Understanding the mechanistic basis of these phenotypes will provide critical information about how nutrient sensing signal transduction pathways contribute to the pathogenesis of adipose tissue related diseases.
Model of mTORC2 signaling in a white adipocyte
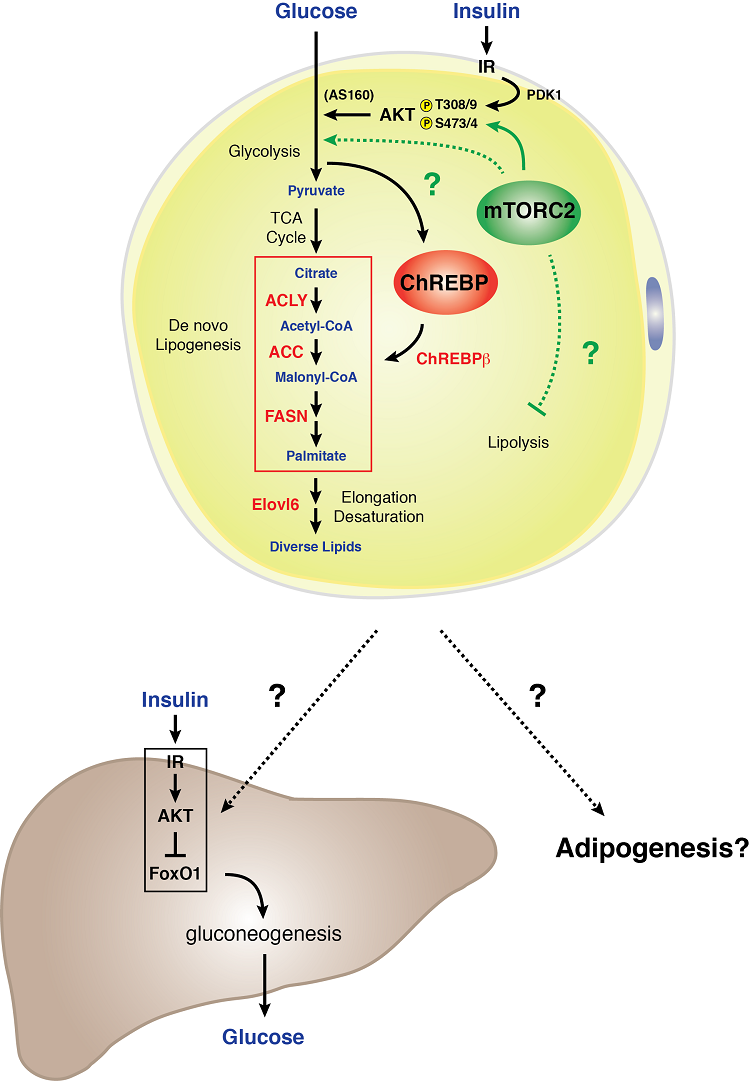 White adipocytes require mTORC2 for normal carbohydrate and lipid metabolism. We found that genetic loss of Rictor (mTORC2) specifically in white adipocytes reduces glucose uptake and attenuates expression of the ChREBP-beta transcription factor and its downstream targets - which includes the enzymes that function in the de novo lipogenesis pathway. In addition, an mTORC2 deficiency in white fat cells results in severe hepatic insulin resistance. These data indicate that white adipocyte mTORC2 is an essential regulator of carbohydrate and lipid metabolism and is a key component of an extra-hepatic nutrient-sensing organ communication mechanism that controls systemic glucose homeostasis [Tang et al., Nature Communications 2016]. Understanding the biology of mTORC2 signaling in white fat may hold important clues to how white adipose tissue functions as a glucose sensing organ and mediator of systemic metabolic fitness.
White adipocytes require mTORC2 for normal carbohydrate and lipid metabolism. We found that genetic loss of Rictor (mTORC2) specifically in white adipocytes reduces glucose uptake and attenuates expression of the ChREBP-beta transcription factor and its downstream targets - which includes the enzymes that function in the de novo lipogenesis pathway. In addition, an mTORC2 deficiency in white fat cells results in severe hepatic insulin resistance. These data indicate that white adipocyte mTORC2 is an essential regulator of carbohydrate and lipid metabolism and is a key component of an extra-hepatic nutrient-sensing organ communication mechanism that controls systemic glucose homeostasis [Tang et al., Nature Communications 2016]. Understanding the biology of mTORC2 signaling in white fat may hold important clues to how white adipose tissue functions as a glucose sensing organ and mediator of systemic metabolic fitness.