Senescence Regulation in Oncogenesis
Tumorigenesis is a multi-step process in which a normal cell acquires changes in a number of critical cancer causing genes. Senescence limits proliferative capacity of cells and thus impedes the accumulation of multiple mutations necessary for tumorigenesis. Furthermore, aberrant oncogenic activation, DNA damage or oxidative stress activates senescence, providing a failsafe mechanism that prevents the proliferation of cells at risk of neoplastic transformation. Emerging evidence supports that senescence is an important tumor suppressor. Consistent with this notion, we have found that Smurf2-deficient mouse embryonic fibroblasts exhibit delayed senescence entry and enhanced potential to become immortalized in culture, while Smurf2-deficient mice show increased susceptibility to various types of cancer, including B-cell lymphoma, hepatocellular carcinoma, adenocarcinoma in small intestine and soft tissue sarcoma. We are generating conditional Smurf2 knockout and transgenic mouse models to dissect the precise mechanism of Smurf2-mediated tumor suppression.
The incidence of cancer increases exponentially with age in human. The age-dependent increase in cancer risk is not well understood. We are interested in whether age-dependent accumulation of senescent cells leads to alterations in tissue microenvironment that is favorable for oncogenesis. Our recent studies as well as others indicate that senescent cells change their secretory patterns of cytokines, chemokines, growth factors, extracellular matrix proteins and proteases. Many of these factors are known to promote tumorigenesis, suggesting that senescence could act as a double-edged sword in both suppressing and promoting tumorigenesis. Using genetically modified mouse models to specifically modulate senescence response in stroma, we examine the possible role of senescent microenvironment in promoting oncogenesis.
 |
|
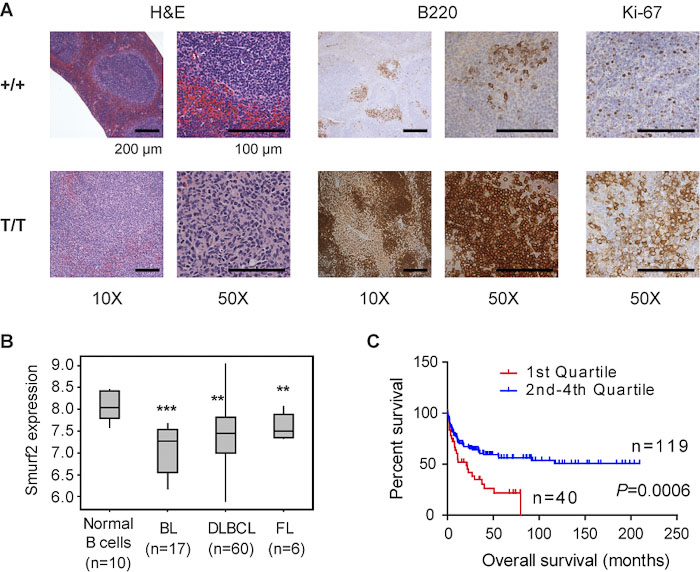
Increase susceptibility to cancer as a result of Smurf2-deficiency. (A) Lymphomas found in Smurf2-deficient mice have the characteristics of diffuse large B-cell lymphoma (DLBCL) and enhanced cell proliferation. (B) Decreased expression of Smurf2 in human Burkitt’s lymphoma (BL), DLBCL and follicular lymphoma (FL). (C) Lower level of Smurf2 expression correlates with poor survival prognosis in patients with DLBCL. |
