Tofacitinib treatment for vitiligo
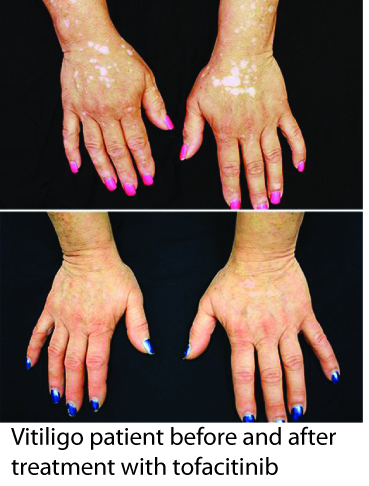 You may have already heard about the recent report by Dr. Brett King at Yale University, where one of his patients with vitiligo responded to treatment with tofacitinib (brand name Xeljanz), regaining much of the pigment in her face and hands within a few months. Tofacitinib is a recently approved drug for rheumatoid arthritis, and acts as a Jak inhibitor, disrupting specific inflammatory pathways that signal through proteins called Jaks, of which there are four - Jak1, Jak2, Jak3, and Tyk2 (I know, Tyk2 should be named Jak4, but we try to keep things interesting by not always following convention when naming proteins :) ).
You may have already heard about the recent report by Dr. Brett King at Yale University, where one of his patients with vitiligo responded to treatment with tofacitinib (brand name Xeljanz), regaining much of the pigment in her face and hands within a few months. Tofacitinib is a recently approved drug for rheumatoid arthritis, and acts as a Jak inhibitor, disrupting specific inflammatory pathways that signal through proteins called Jaks, of which there are four - Jak1, Jak2, Jak3, and Tyk2 (I know, Tyk2 should be named Jak4, but we try to keep things interesting by not always following convention when naming proteins :) ).
While only a single patient, this report is indeed very exciting, as it suggests that Jak inhibitors (there are other related drugs as well) may provide a new effective treatment for vitiligo. We are also excited because Dr. King based his rationale for using tofacitinib in vitiligo on our research, which defined the IFN-g signaling pathway (which requires Jak1 and Jak2) as critical for vitiligo progression and maintenance (read more here). It supports our hypothesis that targeting this pathway (which could be done at multiple different points) would be an effective strategy for developing new treatments.
While this result is exciting for multiple reasons, we must also consider the fact that we don’t have long-term safety data on this drug in humans, and have no data on the safety of tofacitinib in patients with vitiligo. It does increase the incidence of shingles in arthritis patients who take it (about 5% of users), with smaller risks of infection and possibly cancer. So we need to exercise caution when considering it as a treatment. In addition, it is currently only FDA-approved for treating patients with rheumatoid arthritis, and is typically not covered by insurance for vitiligo patients. Since the drug is very expensive (about $30,000 per year), it will not be readily available to most patients for some time. However we hope that producers of newer, “next-generation” versions of this drug might pursue FDA-approval specifically for vitiligo, which should make it more widely available.
So, I think we should all be excited about this report, but remain cautious and hopeful that future developments in vitiligo treatments will be effective, safe, and affordable for those who suffer from this terrible, devastating disease.