Systems Biology of Erythropoiesis
Mammalian red cells have a limited life span, and are therefore generated throughout life, at a rate that equals senescent red cell loss. Erythropoietic rate increases up to ten fold its basal rate in response to a variety of stress conditions, including anemia, bleeding or reduced tissue oxygen tension. Both basal erythropoiesis and the stress response are regulated by a negative feedback loop, where tissue hypoxia stimulates erythropoietin (Epo) secretion, promoting an increase in erythropoietic rate. This well-established long-range negative feedback loop cannot, by itself, account for the observed system properties of erythropoiesis, namely, a wide dynamic range, stability in the face of random perturbations, and a rapid stress response. Our laboratory is interested in determining the molecular circuits that help to control erythropoietic rate and that impart it with these remarkable properties.
We investigated three distinct Epo-regulated erythroblast survival pathways, finding that, in vivo, each gives rise to distinct system properties. We found that the induction of bcl-xL by EpoR-stimulated Stat5 is responsive to the rate of change in Epo blood levels, rather than to its absolute level, and is maximally but transiently activated in acute stress, playing no apparent role in chronic stress. By contrast, Epo -mediated suppression of the pro-survival Fas and Bim pathways is proportional to the absolute levels of Stress/Epo and persists throughout chronic stress (Figure 1; [2]). We suggest that, together, these elements operate in a manner reminiscent of a "PID" feedback controller frequently found in engineering applications (Figure 2; [3]).
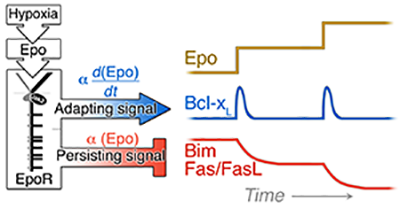
Figure 1: EpoR signaling generates at least two types of signal dynamics: a transient signal in response to changing Epo levels regulates the Bcl-xL pathway; and a persistent signal, responsive to the absolute level of stress, regulates the levels of Fas, FasL and Bim.
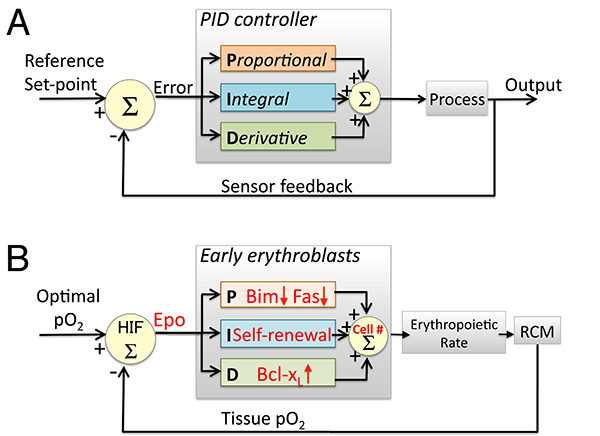
Figure 2: Similarities between feedback regulation of the erythropoeitic system and a PID controller. A: A PID controller is a device that uses feedback to continuously generate the output of a dynamical system so as to match a desired reference value. An 'error' is continuously generated by the comparison of the system's required set-point with the actual value of the parameter controlled by the system. Three elements within the controller generate output that is either proportional ('P'), Integral ('I') or a derivative ('D') function of the error signal. The output of the three elements is then combined ( ∑ ) to generate the system's output. B: The early erythroblast compartment as a PID controller. Epo concentration represents the error signal, generated by the mismatch between the actual and desired oxygen availability, sensed by HIF1 and HIF2. Epo regulates the size of the early erythroblast compartment (the controller 'output') via pathways that use the same 'logic' as those of the PID controller elements. Fas and Bim suppression is proportional ('P') to Epo concentration while regulation of Bcl-xL expression corresponds to the 'D' element since it is a function of the derivative of Epo concentration. Pathways that represent the 'I' element may represent self-renewal of erythroid progenitors. RCM= red cell mass. HIF= Hypoxia Inducible Factor.
We also identified a short-range negative autoregulatory loop within the early erythroblast compartment, operated by Fas/FasL, which filters out random noise and controls a reserve pool of early erythroblasts that is poised to accelerate the response to acute stress (Figure 3 [1, 4, 5]). Of note, both these properties have previously been identified as inherent to negative regulatory motifs in transcriptional circuits, suggesting that circuits of similar 'logic' can regulate larger inter-cellualr systems at the level of the whole organism.
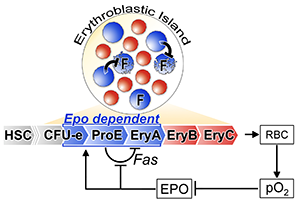 Figure 3: Adjusted feedback diagram of the erythropoietic system. In addition to the long-range Epo/pO2 negative feedback loop, there is a short-range Fas/FasL - mediated negative autoregulatory loop local to the early erythroblast compartment. Epo signaling inhibits local autoregulation by suppressing Fas expression. The illustrated erythroblastic island shows that the frequency of Fas/FasL-expressing early erythroblasts (in blue) within the island determines the probability of their interaction and potential apoptosis. Later erythroblasts (EryB, EryC) are illustrated in red. F=Fas (adapted from [1]).
Figure 3: Adjusted feedback diagram of the erythropoietic system. In addition to the long-range Epo/pO2 negative feedback loop, there is a short-range Fas/FasL - mediated negative autoregulatory loop local to the early erythroblast compartment. Epo signaling inhibits local autoregulation by suppressing Fas expression. The illustrated erythroblastic island shows that the frequency of Fas/FasL-expressing early erythroblasts (in blue) within the island determines the probability of their interaction and potential apoptosis. Later erythroblasts (EryB, EryC) are illustrated in red. F=Fas (adapted from [1]).
References:
1. Koulnis, M., Liu, Y., Hallstrom, K., and Socolovsky, M. (2011). Negative autoregulation by Fas stabilizes adult erythropoiesis and accelerates its stress response. PLoS One 6, e21192.
2. Koulnis, M., Porpiglia, E., Porpiglia, P.A., Liu, Y., Hallstrom, K., Hidalgo, D., and Socolovsky, M. (2012). Contrasting dynamic responses in vivo of the Bcl-xL and Bim erythropoietic survival pathways. Blood 119, 1228-1239.
3. Koulnis, M., Porpiglia, E., Hidalgo, D., and Socolovsky, M. Erythropoiesis: from molecular pathways to system properties. Adv Exp Med Biol. 2014; 844:37-58.
4. Liu, Y., Pop, R., Sadegh, C., Brugnara, C., Haase, V.H., and Socolovsky, M. (2006). Suppression of Fas-FasL coexpression by erythropoietin mediates erythroblast expansion during the erythropoietic stress response in vivo. Blood 108, 123-133.
5. Socolovsky, M., Murrell, M., Liu, Y., Pop, R., Porpiglia, E., and Levchenko, A. (2007). Negative Autoregulation by FAS Mediates Robust Fetal Erythropoiesis. PLoS Biol 5, e252.
