Tuberculosis Pathogenesis
Throughout history, tuberculosis has represented one of the most prevalent and feared diseases of humanity. Despite the development of effective diagnostics and chemotherapy, Mycobacterium tuberculosis still kills more people every year than any other single infectious agent. While the TB control efforts face many socioeconomic challenges, there are also a number of biological features of this disease that make it particularly difficult to control. Chief among these features is the diversity of immunological and pathological outcomes of infection in a natural population. This variability confounds every aspect of TB control: the wide spectrum of pathologies caused by infection confounds diagnosis, microanatomical diversity alters the antibiotic efficacy, and immunological differences between individuals is likely the reason we have been unable to develop a broadly effective vaccine.
The biological basis of this variability has been difficult to address in natural populations because infectious disease outcome is a complex trait that is influenced by a variety of interacting variables (Figure). Recognizing this complexity, our lab has developed unique model systems to investigate each of these variables in an isolated experimental setting. Our approach relies on the incorporating natural and engineered genetic variation in both the pathogen and an experimentally-tractable small animal host. To accomplish this, we have leveraged large multi-parental mouse populations that mirror the genetic diversity of an outbred population, along with phylogenetically diverse collections of Mtb isolates. Using these approaches, we have discovered host genetic loci and immunological features that influence pathogenesis, along with bacterial genes and pathways that determine both virulence and antibiotic efficacy. Current efforts are focused on relating these findings to human patients and further developing them into new interventions. Our group leads an international consortium to pursue this work under the “Systems Genetics of Tuberculosis” Program supported by NIAID.
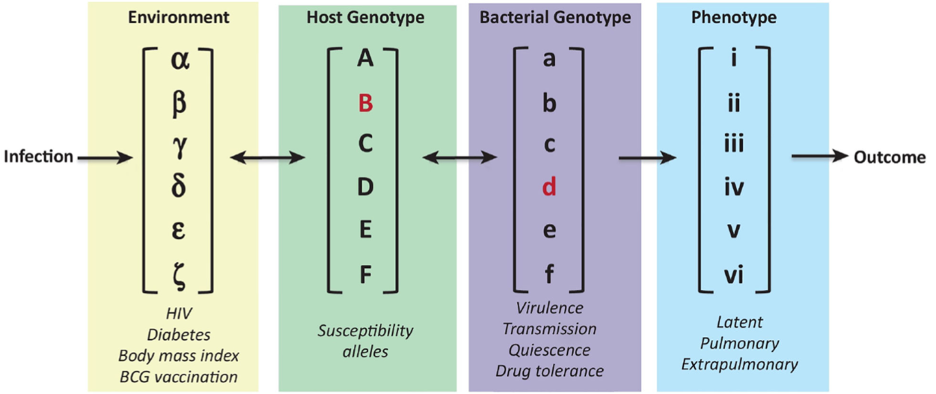
From Smith et al. Trends Microbiol. PMID: 30166218