Bacterial Physiology and Antibiotic Action
Mycobacteria are phylogenetically distinct from the model organisms classically used to study prokaryotic cell biology. Consequently, the mechanisms controlling the growth, division, and morphogenesis of the mycobacterial cell are unique to this genus. As a result, both the targets for effective antibiotics and the mechanisms that lead to resistance are unique to mycobacteria. We are using genetics, genomics and quantitative imaging to understand the mechanisms regulating these processes. This work is currently focused on two major goals:
Understanding the essential processes that control growth and division in mycobacteria, and developing new drugs based on these insights.
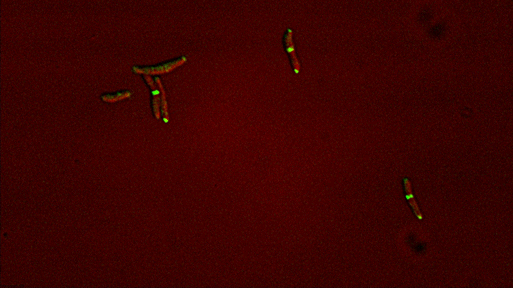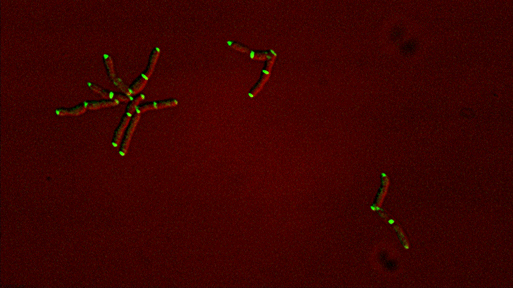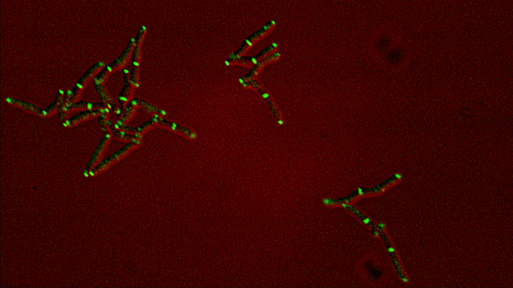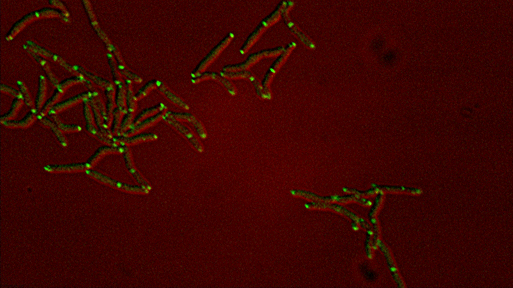
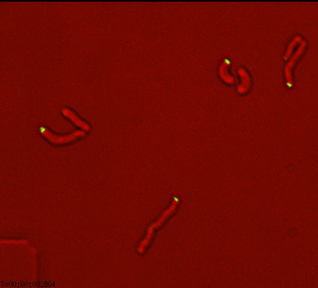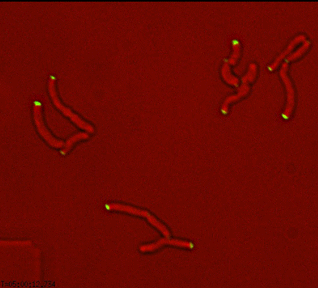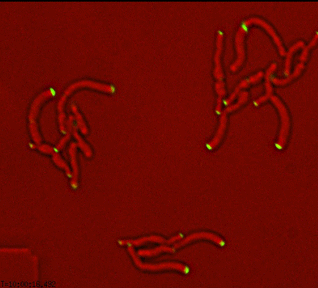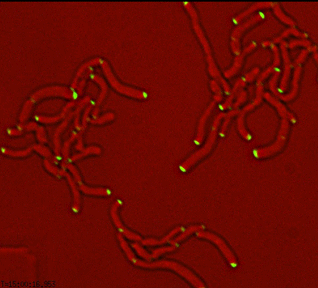
In the past, we have characterized essential phosphosignaling, scaffolding, and regulatory processes that facilitate the unique focal elongation and asymmetric septation processes in mycobacteria. We continue these studies focusing on the spatial coordination of protein complexes and the temporal regulation of gene expression across the cell cycle. These essential functions represent potential targets for new drugs.
|
Dynamic reorganization of cell wall regulatory complexes |
|
 Sequential recruitment of cell wall synthetic and regulatory complexes to the developing septum |
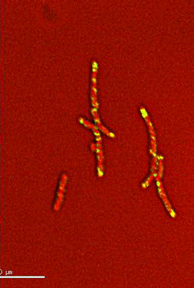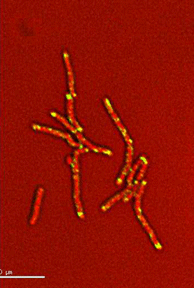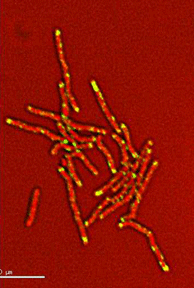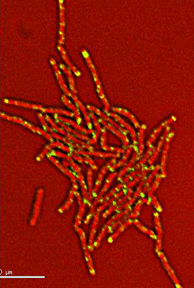 Dynamics of arabinogalactan synthesis during growth |
|
Differential sorting of polar and septal cell wall regulatory complexes |
Morphology mutants of M. smegmatis |
Characterizing the impact of genomic plasticity on the activity of existing drugs and the evolution of resistance.
This work centers on the role of phase variation in the adaptation to stress. The Mtb chromosome contains a significant number of highly-variable simple sequence repeats that are strategically located to alter gene expression, and we have associated several of these with alterations in virulence or antibiotic susceptibility. We use phylogenomic, statistical, and experimental approaches to understand the complex effect of variation in these contingency loci on disease, transmission, and treatment outcome (Figure).
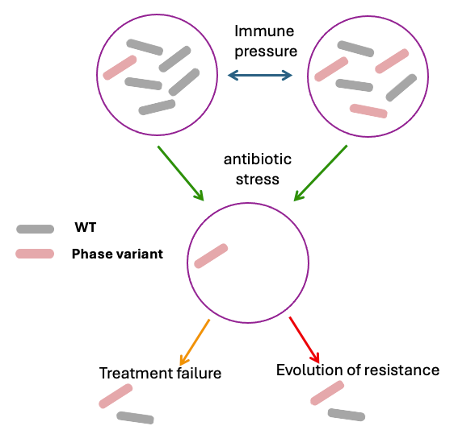
Figure: Possible dynamics controlling the prevalence of phase variants.