Harnessing cellular senescence for cancer immunotherapy
Cellular senescence is a stress-induced process that mediates growth arrest of damaged cells and conveys those damage signals to surrounding cells in microenvironment through a process called the senescence associated secretory phenotype (SASP). The SASP contains many different factors, including inflammatory cytokines and chemokines that can recruit different types of immune cells to target and clear damaged senescent cells. In the setting of cancer, we previously found that SASP induction following therapy-induced senescence can recruit and activate cytotoxic lymphocytes such as Natural Killer (NK cells) and CD8+ T cells in lung and pancreatic tumors, leading to anti-tumor immunity, disease regression, and synergy with checkpoint blockade immunotherapy (Ruscetti et al. Science 2018; Ruscetti et al. Cell 2020). These studies collectively demonstrate that senescence can be a beneficial outcome of cancer therapy and a novel program to reengage anti-tumor immunity in immunologically “cold” tumors, such as pancreas cancer, through its non-cell autonomous effects on the tumor microenvironment.
Despite existing knowledge of the transcriptional and chromatin changes that occur during senescence, it still remains unclear how the senescence program is regulated in different contexts, which factors are important for anti-tumor immune responses, and how expression of these factors may be affected by crosstalk with other cells in the microenvironment. Thus, a deeper understanding of intrinsic and extrinsic senescence regulation is necessary in order to fully harness its tumor suppressive capabilities for cancer therapy. To address these gaps, our laboratory is taking two different approaches:
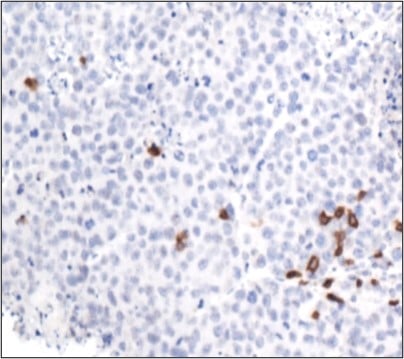
A) Characterizing the tissue-specific regulation of senescence and senescence-mediated immunity
We continue to develop immunocompetent mouse models for mouse and human pancreas cancer, where we can transplant pancreas tumors into different organ systems and subsequently induce senescence with our pharmacological and genetic tools, to assess the impact of distinct tissue microenvironments on SASP factor expression and resulting immune surveillance. By doing so we hope to identify SASP factors that are necessary for immune surveillance and immunotherapy efficacy in pancreas cancer, a disease that is generally resistant to such therapies. Of current interest, we are finding distinct senescence-related chromatin changes in the pancreas tumor microenvironment that may impact anti-tumor immune responses, and are interested in uncovering how cross-talk with other cells in the microenvironment may impact these epigenetic alterations. In the future we hope to apply these approaches to other tumor and tissue contexts to find new therapeutic avenues to stimulate senescence-mediated anti-tumor immunity.
B) Characterizing the impact of distinct genetic drivers on senescence regulation.
In the setting of prostate cancer, we have recently developed a new mouse modeling approach that allows us to flexibly interchange the combinations of oncogenes and tumor suppressors we use to generate tumors (Leibold, Ruscetti et al. Cancer Discovery 2020). We plan to use this system to create genetically defined tumors and cell lines, and then test how the genetics of the tumor dictate responses to senescence-inducing agents and subsequent changes to the immune system and immunotherapy responses.