Structure-based development of inhibitors against the cancer target CtBP
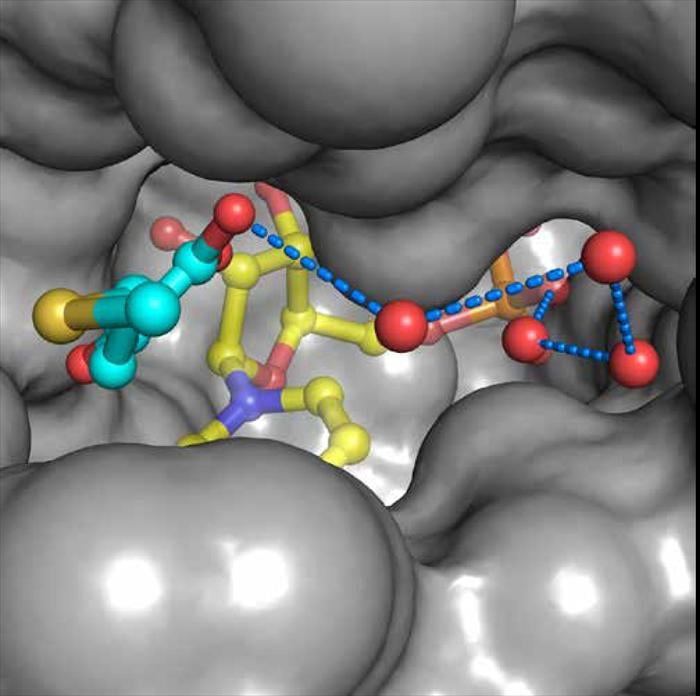
The paralogous transcription coregulators C-terminal Binding Proteins (CtBP) 1 and 2 are critical modulators of numerous cellular processes. CtBP 1 and 2 are overexpressed in many human cancers where they act to inhibit apoptosis and promote metastasis. Unusual among transcription factors, CtBP harbors an essential D-isomer specific 2-hydroxyacid dehydrogenase (D2-HDH) catalytic domain that enables CtBP to be a redox sensor and provides an attractive target for small molecule inhibition. Our collaborator, Dr. Steven Grossman at Virginia Commonwealth University, has shown that high concentrations of substrate are able to inhibit tumor growth in cell culture and mouse studies providing proof-of-principle that CtBP could be an excellent cancer target. We are using structural-based approaches to develop high affinity inhibitors that bind in the substrate pocket (see figure) and can disrupt the oligomeric form required for co-transcriptional activity as potential lead compounds for the development of antineoplastic CtBP inhibitors.