Recent Clinical Trials Conclusively Prove the Efficacy of Endovascular Treatment for Acute Ischemic Stroke Patients
Date Posted: martes, mayo 05, 2015Acute ischemic stroke is the leading cause of disability in the United States with an estimated 800,000 patients suffering a new or recurring stroke annually.[1] Stroke is caused by a blood clot in the arteries of the brain that can lead to paralysis, loss of speech, or death. To date, there are only two treatments for acute ischemic stroke: intravenous tPA (a clot busting drug given intravenously within 4.5 hours of symptom onset) and endovascular intervention that includes mechanical thrombectomy procedure combined with tPA when indicated or without when contraindicated. In addition to the time limitations, intravenous tPA is also contraindicated for patients with an increased risk of bleeding as well as several other comorbid conditions. It has also been shown to be less effective for occlusions that occur in the large arteries of the brain such as at the terminus of the internal carotid artery or middle cerebral artery. Yet these are the patients that will suffer the most debilitating effects of the stroke.
The results of early trials evaluating devices for endovascular stroke therapy did not provide conclusive results. However, after decades of frustration to find new treatments for acute ischemic stroke that improve clinical outcomes, the results of the MR CLEAN, ESCAPE, SWIFT PRIME, and EXTEND IA trials recently published in the New England Journal of Medicine have shown that the addition of endovascular clot removal for the treatment of acute ischemic stroke dramatically improves the rate of good clinical outcome as compared to standard medical care.
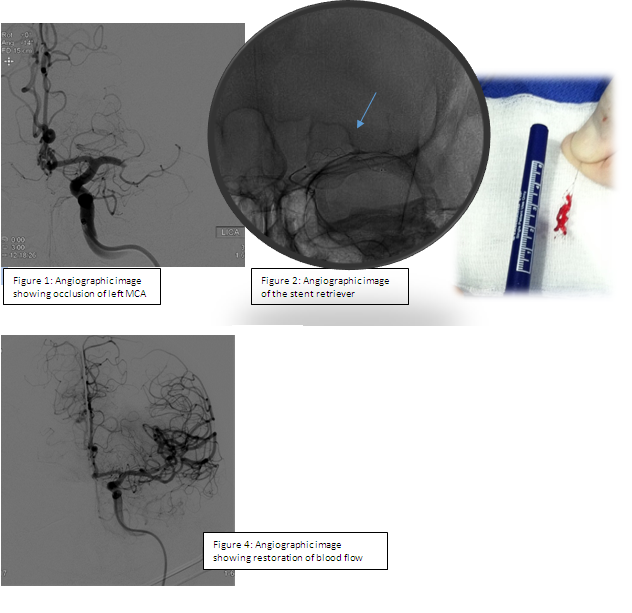
The mechanical thrombectomy procedure involves advancing guidewires followed by catheters into an artery in the groin and up through the neck, until they reach the site of the blood clot causing the stroke. Under angiographic guidance, a stent retriever is then inserted into the catheter and advanced to the site of the occlusion. The stent retriever is allowed to intercalcate with the clot for several minutes and is then carefully withdrawn (Figure 2 below). Follow-up angiographic images are obtained to assess the level of flow restoration as shown in Figure 4 below. If indicated, this procedure may be repeated to fully restore blood flow to the affected area.
 Data from these recent randomized, multi-center stroke trials show that between 33% and 60% of the patients receiving thrombectomy, depending on trial design, were living independently after 90 days.[2-5]This was a statistically significant improvement as compared to standard medical treatment (19-35%). All treatments were administered within 6 to 12 hours of the onset of stroke symptoms, emphasizing the importance of timely medical attention. The US-based SWIFT PRIME trial, which our own neurointerventional team led by Dr. Ajit Puri participated in, showed by far the most promising results with the number of patients needed to treat for a statistically significant improvement in functional outcome in those receiving stent-retriever thrombectomy of 2.6.[4] The number of patients in the mechanical thrombectomy arm needed to treat for functional independence at 90 days was 4. This makes endovascular mechanical thrombectomy the most efficacious treatment of stroke ever described.
Data from these recent randomized, multi-center stroke trials show that between 33% and 60% of the patients receiving thrombectomy, depending on trial design, were living independently after 90 days.[2-5]This was a statistically significant improvement as compared to standard medical treatment (19-35%). All treatments were administered within 6 to 12 hours of the onset of stroke symptoms, emphasizing the importance of timely medical attention. The US-based SWIFT PRIME trial, which our own neurointerventional team led by Dr. Ajit Puri participated in, showed by far the most promising results with the number of patients needed to treat for a statistically significant improvement in functional outcome in those receiving stent-retriever thrombectomy of 2.6.[4] The number of patients in the mechanical thrombectomy arm needed to treat for functional independence at 90 days was 4. This makes endovascular mechanical thrombectomy the most efficacious treatment of stroke ever described.
It is interesting to note that the development of stent retrievers was originally described in experimental models at UMASS Medical School. In 2008, Drs. Wakhloo and Gounis showed that a new device at the time, a closed-cell stent, could be used to capture the clot causing the vessel occlusion and restore blood flow in the vessel.[6] A similar device, later called the stent-retriever, was used in 97%-100% of the patients in the recent randomized trials who actually received the mechanical thrombectomy procedure. UMASS will continue to be in the forefront of this research with future focus on refining the devices and neurointerventional techniques available for mechanical thrombectomy and optimizing the imaging techniques used to identify patients that will most benefit from this treatment.
1. Members, W.G., et al., Heart Disease and Stroke Statistics—2012 Update: A Report From the American Heart Association. Circulation, 2012. 125(1): p. e2-e220.
2. Berkhemer, O.A., et al., A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. New England Journal of Medicine, 2015. 372(1): p. 11-20.
3. Goyal, M., et al., Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. New England Journal of Medicine, 2015. 372(11): p. 1019-1030.
4. Saver, J.L., et al., Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. New England Journal of Medicine. 0(0): p. null.
5. Campbell, B.C.V., et al., Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. New England Journal of Medicine, 2015. 372(11): p. 1009-1018.
6. Wakhloo, A.K. and M.J. Gounis, Retrievable closed cell intracranial stent for foreign body and clot removal. Neurosurgery, 2008. 62(5 Suppl 2): p. ONS390-3; discussion ONS393-4.