Structure and function of myosin binding protein C
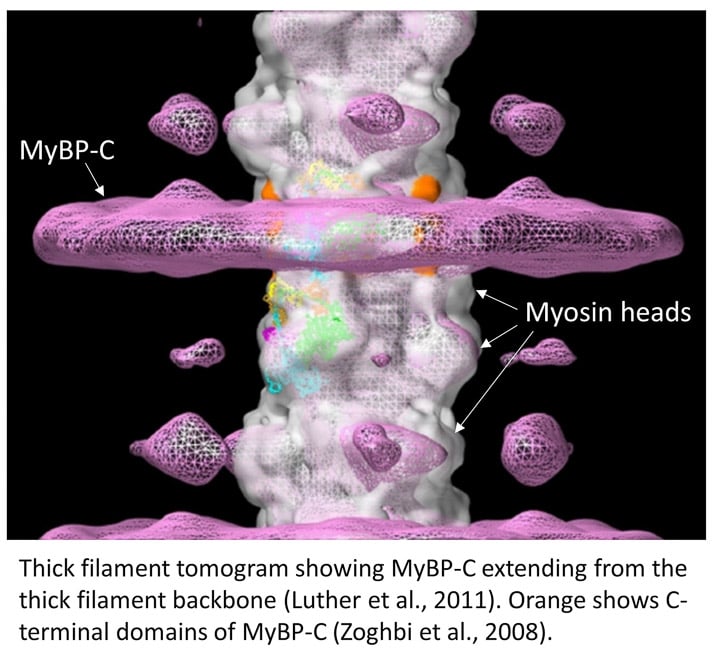
MyBP-C is a myosin binding protein discovered in the 1970s whose structural organization and function have remained elusive. It is currently a topical area of research because of its role in regulating cardiac contraction and because mutations in MyBP-C cause are a major cause of heart (and skeletal muscle) disease. We collaborated with Dr. Pradeep Luther (Imperial College, London) who carried out electron tomography of especially well (cryo) preserved muscle specimens prepared in our lab. The tomograms show that MyBP-C is not only bound to the thick filament, but also extends out and connects to the thin filaments (the pink band of density in the figure; Luther et al., PNAS 2011). This was the first demonstration that interactions of MyBP-C with actin observed in vitro may be relevant to intact muscle. Bridging between myosin and actin provides a physical basis for MyBP-C’s ability to modulate contraction. We are now pursuing further aspects of MyBP-C structure and function by cryo-EM, in collaboration with Drs. David Warshaw at University of Vermont and Richard Moss at University of Wisconsin.