Scientists at UMass Chan Medical School have used a light-operated nanoparticle to remotely and selectively activate T cells engineered and infused into an animal model to attack certain types of tumor cells, such as those found in some leukemia and lymphoma cancers, according to new research published in Nature Nanotechnology.
This nano-optogenetic engineered immunotherapy provides better and safer control of the immune response in patients than currently available cancer-fighting immunotherapies, therefore allowing further refinement of personalized anti-cancer therapy for next-generation precision medicine.

“Our efforts are contributing to better and safer CAR T cell immunotherapy,” said Gang Han, PhD, professor of biochemistry & molecular biotechnology. “We believe that this nano-optogenetic immunotherapy represents a new generation of personalized cancer treatment, in which precise control of the timing, location and dosage of T cell activation could be tailored to an individual patient. It could also have applications for providing finer control of emerging cellular immunotherapies based on natural killer cells and macrophages.”
Chimeric antigen receptor (CAR) T cell therapy is a type of immunotherapy in which T cells derived from a patient are engineered to express an artificial protein on the cell surface that can target antigens from a patient’s tumor cells. After these engineered T cells are re-infused into a patient, they attack and kill the cancer cells. The first CAR-T cell therapies were approved by the FDA in 2017. There are now six approved CAR-T therapies for cancers such as diffuse large B-cell lymphoma and B-cell acute lymphoblastic leukemia.
Despite this success, the safety issue of “on-target, off-tumor” cytotoxicity in CAR T immunotherapy has caused severe side effects for some patients. In these cases, uncontrolled CAR T cells attack healthy cells or organs expressing the specific CAR antigen and could induce B cell aplasia. Moreover, some of the treatment receivers have experienced cytokine release syndrome, which could be life-threatening, said Dr. Han.
To help control the immune response on CAR T cell therapy, Han, in collaboration with Yubin Zhou, PhD, associate professor at the Texas A&M Health Science Center, has taken a light-activated gene from the oat plant and developed a nano-optogenetics dual edited light-switchable chimeric antigen receptor (LiCAR) T cell system that can be remotely activated and spatiotemporally controlled by near-infrared light. This team genetically engineered the functional domains of a chimeric antigen receptor into two splits and installed photo-responsive modules on each split. The two modules, consisting of Avena light-oxygen-voltage domain 2 (LOV2) and its binding partner sspB (the LOV2-ssrA/sspB pair) from the oat plant, complete the assembly of the split CAR into a functional CAR only when exposed to blue light, much like how a switch in a person’s home turns on a light by completing an electrical circuit.
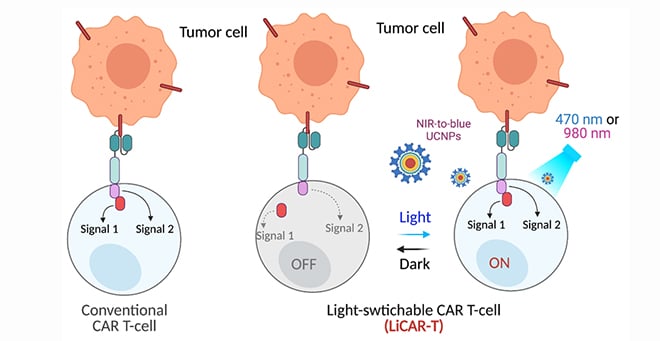
the LiCAR T-cells by NIR-to-blue upconversion luminescence.
However, to activate the LiCAR T the light needs to penetrate through tissue. To address the issue of limited tissue penetration, Han employed a special type of luminescent nanomaterial, called upconversion nanoparticles (UCNP). These tiny nanomaterials capture near-infrared light, which is able to penetrate several centimeters into the body, and convert that light to emit a bright blue light that in turn activates the LiCAR T.
In a mouse model of a CD19 positive tumor, Han demonstrated that the LiCAR T cell/UCNP system responded specifically to CD19 positive tumors and only at the tumor site when exposed to NIR irradiation. To confirm the enhanced safety of the system, “on-target, off-tumor” cytotoxicity was monitored. In contrast to the standard CAR T cell therapy, which induced significantly decreased B cells and dramatic elevation of IL-6 cytokine in mice, the LiCAR T cell/UCNP treated mice showed a similar level of B cells and IL-6 compared to healthy mice.
“Taking advantage of both the NIR-to-blue nanophotonics and genetically engineered LiCAR T, we could accomplish ano-optogenetic immunotherapy wirelessly controlled in a fine spatiotemporal manner, and therefore secure a safer and personalized cancer treatment in mice,” said Han.
“We believe that such safety challenges of the standard CAR T immunotherapy could be addressed by precise control of the immune response of the CAR T cells, thus making the CAR T cells functioning at specific timing and location on-demand. In this way, the ‘on-target, off-tumor’ cytotoxicity and systematic CRS could be avoided by initiating the CAR T activations only in the tumor,” Han added.
Related stories on UMassMed News:
Gang Han optogenetic research named a top paper of 2019 by Cell
Gang Han receives UMass Tech seed funding to explore safer class of MRI contrast agents
Gang Han, colleagues develop tumor-targeting MRI contrast based on human protein