Health care workers across the nation this week are the first Americans to receive the new mRNA vaccine by Pfizer/BioNTech to protect against COVID-19, and the Moderna vaccine appears soon to follow, with emergency approval by the Food and Drug Administration possible any day. The new mRNA vaccines are the first of their kind and researchers at UMass Medical School are among the leading RNA biologists in the world. The Medical School is home to the RNA Therapeutics Institute, which employs nucleic acid scientists and clinicians to create a new paradigm for organizing molecular research that enables the rapid application of new biological discoveries to solutions for unmet challenges in human health.
Here’s a primer on how mRNA vaccines work.
|
|

|
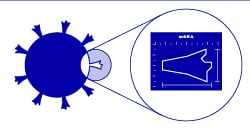
The immune system mobilizes and records the shape of the SARS-CoV-2 protein. |
|
Inside our cells, the DNA sequences that hold the instructions for each of the roughly 20,000 protein-coding genes in the human genome are safely sequestered inside a cell’s nucleus for protection. These sequences of nucleotides that make up our genes hold the blueprints for building proteins, the long chains of amino acids that perform the basic functions of life inside a cell, including DNA replication, responding to stimuli, transporting molecules, performing metabolic activities and building cell structures. Because DNA is so fragile and important, however, an intermediary needs to move this information from the nucleus of the cell, where the genome resides, to the cytoplasm, where the amino acids and molecules that are the building blocks to make these proteins are found.
messenger RNAs (mRNA) are the set of protein-building instructions that can move from the nucleus of the cell to the cytoplasm. mRNAs are created as an exact copy of the segment of DNA found along the genome corresponding to a protein-coding gene. Unlike DNA, the mRNA can move from the cell’s nucleus to the cytoplasm. Once inside the cell’s cytoplasm, the machinery responsible for building proteins, called the ribosome, reads the mRNA and gets to work making proteins. After the mRNA is read and the building process begins, the mRNA is quickly destroyed by the cell. Destruction of the mRNA ensures that the cell does not make too much of one type of protein.
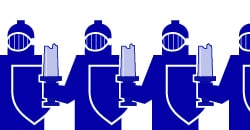
Scientists are using the mRNA process to develop vaccines against viruses such as SARS-CoV-2. When a virus infects a cell, it uses the cell’s protein-making machinery to make its own proteins instead of the cell’s proteins. It then uses these viral proteins to make more of itself, replicate and infect even more cells. The immune system is designed to recognize these foreign proteins, wake up and defend itself.
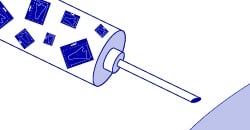
The idea behind mRNA vaccines, like the kind developed by Moderna and Pfizer for COVID-19, is to insert an mRNA from the virus into a cell. The cell would then turn this mRNA into a viral protein. A single viral protein would not be enough to cause the cell harm or the host to become sick. Given the right mRNA and the right protein, however, it may activate the immune response. In this case, the immune system could begin mounting a response and deploying antibodies against the virus without being infected. The body would then be able to easily fight off any subsequent infection quickly because antibodies are already present, eliminating or mitigating the severity of the illness.
UMass Medical School is home to some of the foremost RNA biologists in the world. In 2006, Craig Mello, PhD, Howard Hughes Medical Institute Investigator, the Blais University Chair in Molecular Medicine and distinguished professor of RNA therapeutics and molecular medicine, was honored with the Nobel Prize in Medicine or Physiology for his co-discovery of RNAi, a natural molecular process inside cells that uses small RNA molecules to halt the translation of genes into proteins. These small RNA molecules effectively turn a gene off by stopping the protein-building machinery inside the cell—a process scientists call silencing.
A leader in RNA science, Phillip D. Zamore, PhD, Howard Hughes Medical Institute Investigator, the Gretchen Stone Cook Chair of Biomedical Sciences and chair and professor of RNA therapeutics, identified the biochemical machinery responsible for RNA silencing, the mechanism through which small pieces of genetic material can turn specific genes on or off.
Victor R. Ambros, PhD, the Silverman Chair in Natural Sciences and professor of molecular medicine, was co-recipient of the 2008 Albert Lasker Award for Basic Medical Research for his co-discovery of microRNA, the very short, single-stranded RNA molecules that are now understood to play a critical role in gene regulation.
Meanwhile, UMMS scientists Anastasia Khvorova, PhD, the Remondi Family Chair in Biomedical Research and professor of RNA therapeutics, and Jonathan K. Watts, associate professor of RNA therapeutics and biochemistry & molecular pharmacology, seek to turn these natural RNA molecules into a powerful therapeutic for treating human disease.
Related stories on UMassMed News:
NIH research funding surges at UMass Medical School
UMass Medical School receives $100 million in NIH grants to lead push for fast, accessible COVID-19 tests
UMass Medical School researchers visualize new states of ribosome translation with cryo-EM
Katherine Fitzgerald and Anastasia Khvorova named Harrington Scholars