The RTI Takes on the Coronavirus
Khvorova and Watts labs use RNAi to develop COVID-19 therapeutics. Read more.
Medicinal Chemistry of Therapeutic RNAs
RNA interference provides researchers with a simple and effective tool to inhibit the function of any human gene. The inherent sequence specificity and lasting activity of small RNAs makes them ideal drugs that are expected to transform drug development and our approach to human health. The promise of RNA interference as a general therapeutic strategy, however, will depend on the ability to deliver small RNAs to a wide range of tissues. We believe that modulation of RNA chemistry is the way to enable delivery to wide range of tissues. A recent breakthrough in conjugate-mediated delivery of siRNA was the development by Alnylam Pharmaceuticals of siRNA conjugated to GalNAc (triple n-acetylgalactosamine), which drives efficient, receptor-mediated uptake by hepatocytes, with a promise of robust clinical efficacy with quarterly or even every half-year injections. Thus, the challenge of liver delivery is solved – and the rest of the body is open for chemical exploration. Our target is to develop chemistries that will enable oligonucleotide delivery to other tissues, with properties comparable to that of GalNAcs for Hepatocytes.
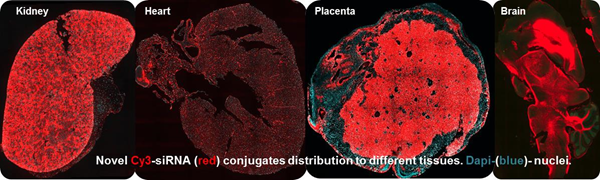
The focus of the Khvorova Lab is enabling therapeutic oligonucleotide delivery to tissues other than liver through chemical engineering. We are interested in identifying chemical and biological properties that drive small RNA tissue distribution, retention, cellular uptake, and biological availability. By screening a wide range of chemically engineered and naturally occurring bioactive conjugates, we already have identified novel chemical modalities that support delivery of robust amounts of RNAs to the heart, kidneys, muscle, placenta, vasculature, and brain, tissues previously untargetable by RNAi.






