Research Interests
Chronobiology:
 Our environment is constantly changing. The Sun rises and sets every day, causing rhythmic changes in light and temperature. At most latitudes, weather and day length vary as seasons pass. On the coastline, tides rise and fall. Because these environmental cycles occur with precise periodicities, most organisms on Earth have acquired biological clocks that can track and "predict" them. Organisms can thus adapt and anticipate changes in light intensities, temperature, day length or water levels by adjusting their physiology and behavior in a time-dependent manner. Circadian clocks have a period of 24 hours, and synchronize to daily environmental cycles. They regulate metabolism and physiology throughout our body, and their disruption - for instane by shift work - can have serious detrimental health conse
Our environment is constantly changing. The Sun rises and sets every day, causing rhythmic changes in light and temperature. At most latitudes, weather and day length vary as seasons pass. On the coastline, tides rise and fall. Because these environmental cycles occur with precise periodicities, most organisms on Earth have acquired biological clocks that can track and "predict" them. Organisms can thus adapt and anticipate changes in light intensities, temperature, day length or water levels by adjusting their physiology and behavior in a time-dependent manner. Circadian clocks have a period of 24 hours, and synchronize to daily environmental cycles. They regulate metabolism and physiology throughout our body, and their disruption - for instane by shift work - can have serious detrimental health conse quences. Circatidal clocks are found in coastal organisms. They have a period of 12.4 h and synchronize to tides. Our lab’s overall objective is to elucidate the molecular and neural mechanisms that underlie these biological rhythms, and understand how biological clocks allow for behavioral adaptations to environmental cycles. Most of our work is performed in Drosophila melanogaster, a fantastic model organism to study both the fundamental mechanisms underlying circadian rhythms and one of its critical outputs: sleep. We have also recently begun to use Parhyale hawaiensis, a genetically-tractable crustacean, to study circatidal clocks, the mechanisms of which are very poorly understood.
quences. Circatidal clocks are found in coastal organisms. They have a period of 12.4 h and synchronize to tides. Our lab’s overall objective is to elucidate the molecular and neural mechanisms that underlie these biological rhythms, and understand how biological clocks allow for behavioral adaptations to environmental cycles. Most of our work is performed in Drosophila melanogaster, a fantastic model organism to study both the fundamental mechanisms underlying circadian rhythms and one of its critical outputs: sleep. We have also recently begun to use Parhyale hawaiensis, a genetically-tractable crustacean, to study circatidal clocks, the mechanisms of which are very poorly understood.
 Circadian Rhythms in Drosophila:
Circadian Rhythms in Drosophila:
Our lab has had a long interest in the molecular and neural mechanisms underlying circadian rhythms and their entrainment. Circadian rhythms are generated in Drosophila by a negative transcriptional feedback loop with a 24 h period, in which two key proteins, PERIOD (PER) and TIMELESS (TIM) negatively regulate their own gene expression through inhibition of the CLOCK/CYCLE (CLK/CYC) heterodimeric transactivator. Kinases and phosphatases add additional layers of temporal and spatial specificity by regulating PER and TIM stability and nuclear entry. Importantly, this feedback loop type of mechanism, shown below in Drosophila, is highly conserved throughout the animal kingdom.
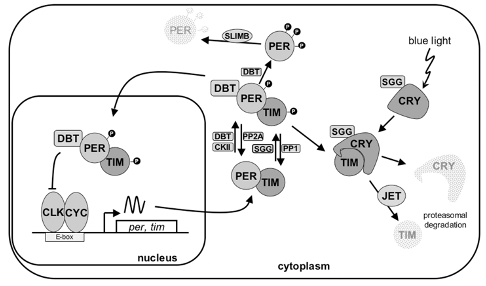
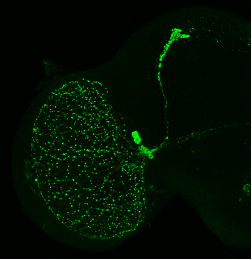
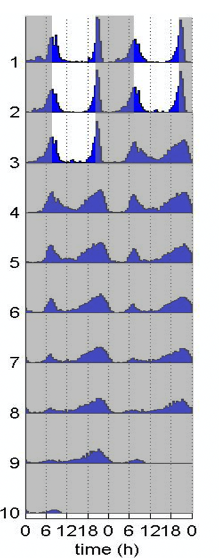 Robust circadian behavioral rhythms of activity and rest can be observed after Drosophila are "entrained" to circadian cycles (wild-type fly circadian locomotor behavior is shown on the double-plotted actogram, right). These are generated by a network of specific neurons in the fly brain. Among them, the ventral lateral neurons (in green, below) are particularly important, as they rhythmically release the neuropeptide PDF and other neurotransmitters to synchronize the other circadian neurons of the fly brain, and thus behavior.
Robust circadian behavioral rhythms of activity and rest can be observed after Drosophila are "entrained" to circadian cycles (wild-type fly circadian locomotor behavior is shown on the double-plotted actogram, right). These are generated by a network of specific neurons in the fly brain. Among them, the ventral lateral neurons (in green, below) are particularly important, as they rhythmically release the neuropeptide PDF and other neurotransmitters to synchronize the other circadian neurons of the fly brain, and thus behavior.
We have studied in detail how light is sensed by the Drosophila circadian clock through the intracellular photoreceptor Cryptochrome (CRY), which triggers the degradation of the circadian pacemaker protein TIM, and thus subsequently PER. Strikingly, this cell-autonomous light detection occurs in most organs, including in the clock neurons that control circadian behavior. However, we have also found that in the brain, network interactions between circadian neurons are critical for proper circadian light responses. Our lab has also been very interested in understanding the role of both RNA binding proteins (such as ATX2 and PSI) and miRNAs in the control of circadian behavior. Finally, we have recently begun to work on a poorly characterized, but critical property of biological clocks: their temperature compensation, which ensures a steady (and thus reliable) circadian period over a wide range of ambient temperatures.
Drosophila sleep:
Sleep is likely the first circadian output you think of. Most humans wake up and go to bed at specific times that are driven by our circadian clock. Though the importance of sleep for our health and survival is well established, its mechanisms and the functions are still poorly understood. Our lab is interested in understanding both homeostatic and circadian mechanisms underlying sleep. We are particularly focused on the role of glia in sleep regulation. We have recently identified the astrocytic GABA transporter GAT as a key regulator of sleep, through modulation of the activity of arousal-promoting circadian neurons.
Circatidal rhythms in Parhyale hawaiensis:
The intertidal zone along ocean coastlines is a particularly complex environment; not only do animals have to cope with seasonal and daily environmental cycles, but additionally they face changes in water levels, with changes in water height that can reach up to 16 meters in some areas. It has been known since the early 20th century that animals are able to anticipate tides, but the mechanisms underlying the circatidal clock are still unknown. This is in large part because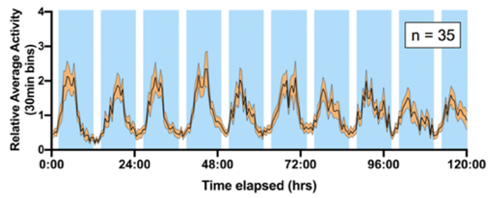 there has not been a fully genetically-tractable model organism to study circatidal rhythms. We have now established that Parhyale hawaiensis, an organism amenable to transgenesis and CRISPR/Cas9-mediated genome editing, expresses circatidal rhythms of swimming (shown on the graph on the left), that can be entrained to artificial tides in the lab. We are therefore now poised to elucidate the mechanisms underlying circatidal rhythms and their entrainment.
there has not been a fully genetically-tractable model organism to study circatidal rhythms. We have now established that Parhyale hawaiensis, an organism amenable to transgenesis and CRISPR/Cas9-mediated genome editing, expresses circatidal rhythms of swimming (shown on the graph on the left), that can be entrained to artificial tides in the lab. We are therefore now poised to elucidate the mechanisms underlying circatidal rhythms and their entrainment.