Bosco Lab Research

Research Overview: mechanisms of neurodegeneration
Our lab is investigating the pathogenic mechanisms associated with neurodegenerative diseases, namely ALS (amyotrophic lateral sclerosis), also known as Lou Gehrig’s disease, and frontotemporal dementia (FTD). We use a multidisciplinary approach involving protein biochemistry, structural biology, human stem cell technology (to generate human motor neurons and human microglia), biophysics and mouse model systems for our investigations. Lab members are welcome to focus on one or multiple disciplines for their research. Our projects are both basic-science and translationally oriented, as our ultimate goal is to translate our basic-research findings into therapies for these devastating diseases.
Protein misfolding in ALS/ FTD
 Many neurodegenerative disease-associated proteins become misfolded as a consequence of genetic mutations and/or altered post-translational modifications. Protein misfolding can lead to gain of toxic function or loss of normal function, and generally results in pathological protein aggregation. Our lab works towards defining the misfolded conformation(s) associated with pathogenic forms of SOD1 (Bosco, Nature Neuroscience, 2010; Rotunno, JBC, 2014), profilin-1 (Boopathy, PNAS, 2015; Schmidt, PNAS, 2021) and FUS/TLS (Sama, Sci Rep, 2017) using biochemical and biophysical methods. We use this information to screen for small-molecules (i.e., pharmacological chaperones) and to develop biologics against toxic, misfolded proteins.
Many neurodegenerative disease-associated proteins become misfolded as a consequence of genetic mutations and/or altered post-translational modifications. Protein misfolding can lead to gain of toxic function or loss of normal function, and generally results in pathological protein aggregation. Our lab works towards defining the misfolded conformation(s) associated with pathogenic forms of SOD1 (Bosco, Nature Neuroscience, 2010; Rotunno, JBC, 2014), profilin-1 (Boopathy, PNAS, 2015; Schmidt, PNAS, 2021) and FUS/TLS (Sama, Sci Rep, 2017) using biochemical and biophysical methods. We use this information to screen for small-molecules (i.e., pharmacological chaperones) and to develop biologics against toxic, misfolded proteins.
Techniques: protein purification, biochemical assays, immortalized cell culture, cloning.
Stress granules and impaired cellular stress response in neurodegenerative disease
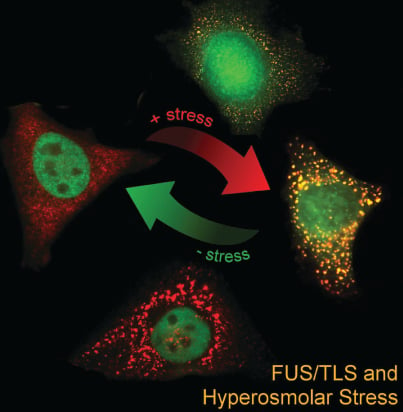 ALS/FTD-associated RNA-binding proteins have been found to associate with stress granules, cytoplasmic RNA granules that form in response to stress. For example, we showed that ALS-linked FUS robustly associates with stress granules (Bosco, Hum Mol Genet, 2010) and that mutant FUS alters the functional properties of these granules (Baron, Mol Neurodegen, 2013; Baron, Hum Mol Genet, 2019). Interestingly, the ALS/FTD-associated RNA-binding proteins FUS and TDP-43 respond to various types of stress by translocating from the nucleus into the cytoplasm (Sama, J Cell Physiol, 2013 *cover story; Tischbein, JBC, 2019). Our current efforts are focused on: 1) identifying the RNAs regulated (or dysregulated) by these proteins under stress; 2) investigating stress granules in vivo; and 3) studying the effects of physiological forms of stress, such as traumatic brain injury (TBI), on neurodegeneration.
ALS/FTD-associated RNA-binding proteins have been found to associate with stress granules, cytoplasmic RNA granules that form in response to stress. For example, we showed that ALS-linked FUS robustly associates with stress granules (Bosco, Hum Mol Genet, 2010) and that mutant FUS alters the functional properties of these granules (Baron, Mol Neurodegen, 2013; Baron, Hum Mol Genet, 2019). Interestingly, the ALS/FTD-associated RNA-binding proteins FUS and TDP-43 respond to various types of stress by translocating from the nucleus into the cytoplasm (Sama, J Cell Physiol, 2013 *cover story; Tischbein, JBC, 2019). Our current efforts are focused on: 1) identifying the RNAs regulated (or dysregulated) by these proteins under stress; 2) investigating stress granules in vivo; and 3) studying the effects of physiological forms of stress, such as traumatic brain injury (TBI), on neurodegeneration.
Models: iPSC-derived neurons and ALS/FTD mouse models.
Techniques: Tissue and cell staining (microscopy), RNASeq, intravital imaging.
Modeling ALS/FTD with human induced pluripotent stem cells (iPSCs)
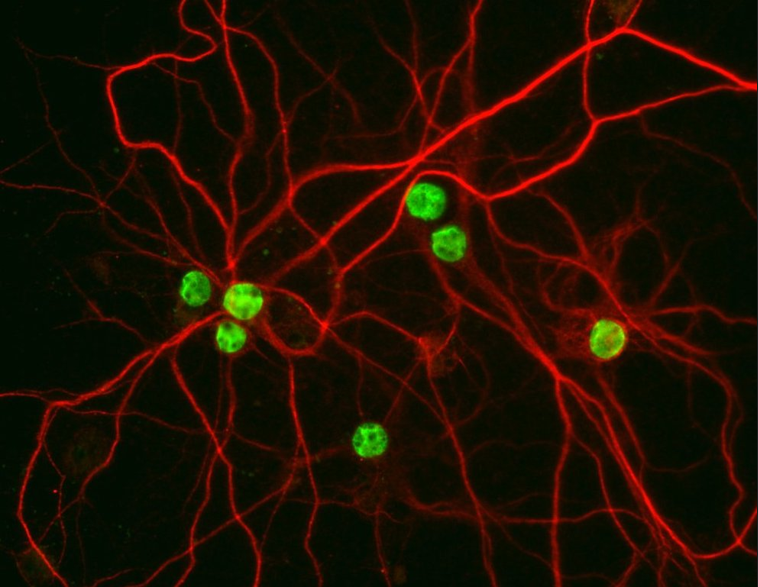 We are currently studying mechanisms of ALS and FTD with patient derived human iPSCs. Mutations are corrected or introduced using CRISPR/Cas9, resulting in isogenic lines for our studies. Using iPSC-derived neurons, we found that mutant FUS causes defects in the nucleocytoplasmic transport pathway (Lin, Nature Neuroscience, 2021).
We are currently studying mechanisms of ALS and FTD with patient derived human iPSCs. Mutations are corrected or introduced using CRISPR/Cas9, resulting in isogenic lines for our studies. Using iPSC-derived neurons, we found that mutant FUS causes defects in the nucleocytoplasmic transport pathway (Lin, Nature Neuroscience, 2021).
We are also differentiating iPSCs into microglia. As the video (left) demonstrates, microglia are motile cells that phagocytose material. In the video, the phagocytosed material becomes fluorescent when it enters the phagolysosomal pathway.