Industry Challenges and Our Solutions
There are many industrial challenges and hurdles that the Autoimmune Therapeutics Institute hopes to create solutions for through our research, studies, and future findings. Below is a list of current existing challenges within the industry and our proposed solutions on how to remedy those hurdles.
Large gap between basic science and clinical understanding
- What is relevant to transform clinical care?
- Observations in clinic (bedside) are not communicated to the researchers (bench) to support new ideas
- Lack of expertise to learn from clinical studies at early stages of mechanistic disease research
Solution
AITI Director and faculty are experienced physician-scientists who treat patients with disease and direct research on that disease.
Difficult to find disease-relevant targets
- Differences between humans and animal models
- Complexity of cell-cell interactions and communications in autoimmunity
Solution
Cutting-edge technologies using multiomics provides disease-relevant, high-resolution data that map complex cellular interactions through signaling that can be targeted therapeutically.
Difficult to access affected human tissues
- Sampling of joints, lungs, kidney for lupus difficult and cause morbidity
- Pancreas in T1D nearly impossible due to volatility of organ, nPOD attempts to solve this by collecting cadaveric organs of limited value to target discovery
- Brain in MS nearly impossible to access in live patients
Solution
Skin is an excellent model organ that is affected by all types of diseases in other organs. It is highly accessible in human patients to analyze cells, signals, disease processes directly ex vivo. The data gathered from skin diseases will be applied as a template to accelerate discoveries in less-accessible organs, which will then support additional progress in a cross-discovery and cross-validation strategies.
Information about diseases remains siloed within organ systems
- Medical specialties were defined over a century ago, based on antiquated view of medicine (procedure-based, not disease pathogenesis-based)
- Departments create boundaries that inhibit collaboration, money flow, leadership vision
Solution
Physician scientists from multiple medical specialties are housed under one roof, with scheduled interactions through shared lab meetings, shared lab space, shared resources, and a single leadership vision; there is strong support from institutional leadership for this unconventional cross-disciplinary model; multiomic analyses are performed by an experienced, central team who will integrate data across diseases.
Efficient approach to target selection is not well-defined
- Which differentially expressed genes are disease-drivers?
- Which disease drivers can be targeted by small molecules/antibodies/siRNA?
Solution
We will use advanced computational biology and existing high-resolution datasets from existing drug mechanisms as well as disease mechanisms to develop a target discovery algorithm that predicts optimal drug targets for further development.
Lack of technical expertise of researchers in drug development, incentives to develop their ideas
- How do we convert mechanistic understanding to a new drug?
- How do we select diseases and drugs that have market potential?
- How do we navigate unmet need into commercial opportunity?
- Details about intellectual property and regulatory issues are unclear to researchers
- Scientists are often not business-savvy about market equity, investments, etc
- Know-how to negotiate contracts is not frequently taught to researchers
- Opportunities for investments are largely relationship-based, faculty do not have access to key stakeholders in drug development
Solution
Leadership of the AITI has direct experience in drug development, biotech startup, and commercialization; we will leverage existing relationships with key representatives at all levels to begin conversations about commercialization; Advisory board members participate in this process at every level (pharma CEO/CSO/CMO, venture partners, regulatory representatives, successful scientists) to support decision-making in target selection and drug development, as well as licensing to interested parties; business support structures at UMass Chan will support AITI and has successfully licensed funding of new discoveries that have entered clinical trials and commercialization.
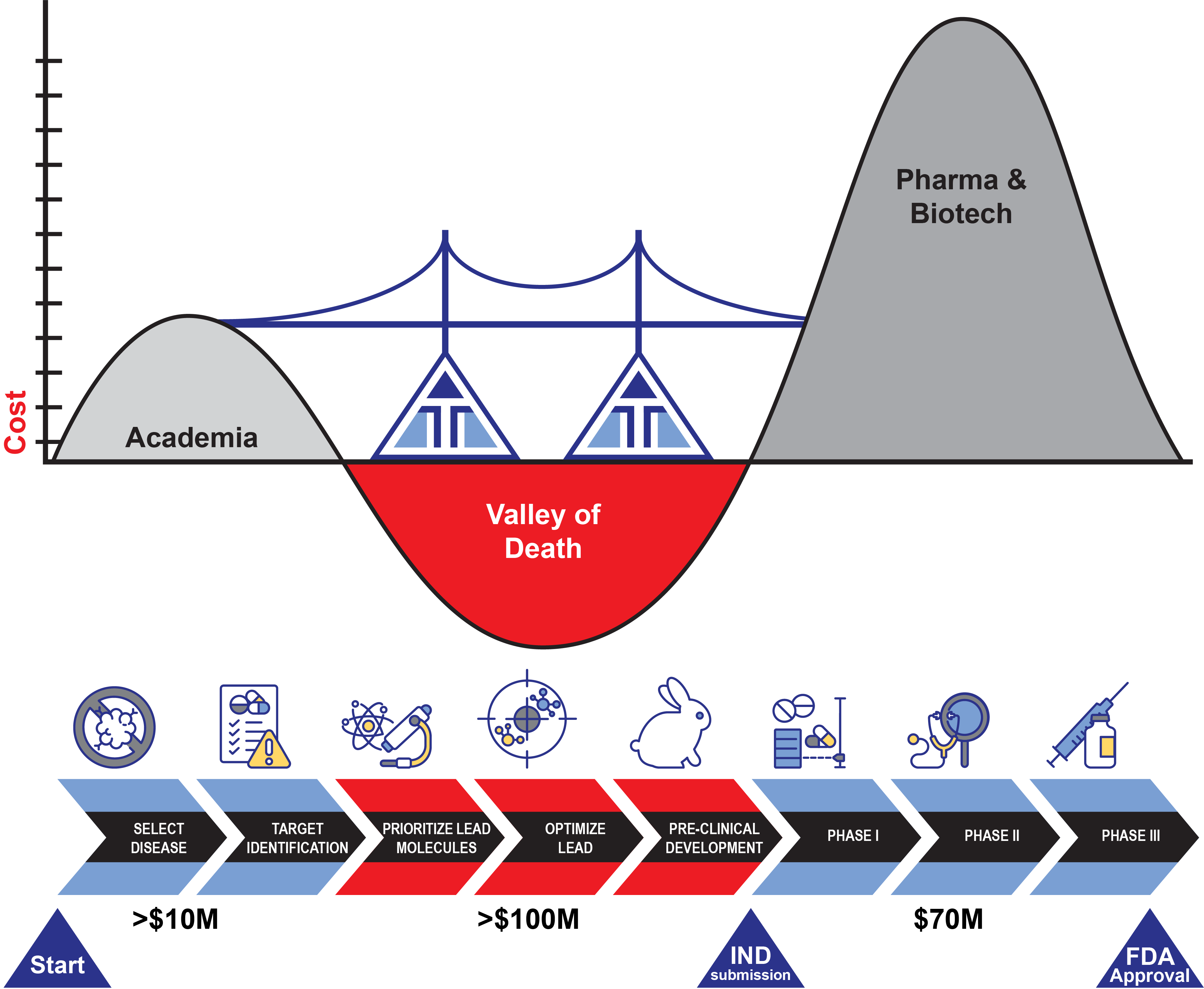
Valley of Death represents the funding gap between preclinical basic discoveries and actual drug creation and testing in early clinical trials
- NIH funds mechanistic research, not drug development
- Major investments from venture and pharma require de-risking through late preclinical/early clinical stage
Solution
We will leverage creative and innovative funding mechanisms that bridge the valley of death through venture philanthropy, NIH funding of early clinical studies, early venture investments (angel-like), and early nonexclusive engagement with pharmaceutical industry; GMP manufacturing capabilities exist at UMass Chan for small POC trials with targeted siRNA.
The AITI has raised the amounts shown below (>$10M, >$100M)