Modeling and Studying the Autoimmune Processes of Human Type 1 Diabetes Using Human Cells and Tissues
Animal models of Type 1 diabetes (T1D) have provided valuable insights for decades, however rodent experiments have not provided an understanding of the root cause of how the disease develops in humans. The research team at the UMass Chan Diabetes Center of Excellence (DCOE) continues to gain new knowledge of T1D by recreating human immune systems inside of unique “humanized” mice to investigate human cells and tissues.
A manuscript published in Molecular Metabolism (February 2022) emphasizes the concept that T1D pathogenesis is far more heterogeneous than has previously been widely appreciated by the broad diabetes research community.
“To understand the role of genetic and environmental factors or to test potential therapies in humans, T1D models must be developed in which human cells can be manipulated and their functions studied under conditions that mimic as closely as possible the physiological conditions of the human body,” said David Harlan, MD, the William and Doris Krupp Professorship of Medicine and co-director of the Diabetes Center of Excellence at UMass Chan Medical School.
The DCOE research team has enjoyed a long and fruitful relationship with colleagues at Vanderbilt University and other institutions, studying pancreatic islets isolated from individuals who have died after being diagnosed with T1D for variable periods prior to their terminal event.
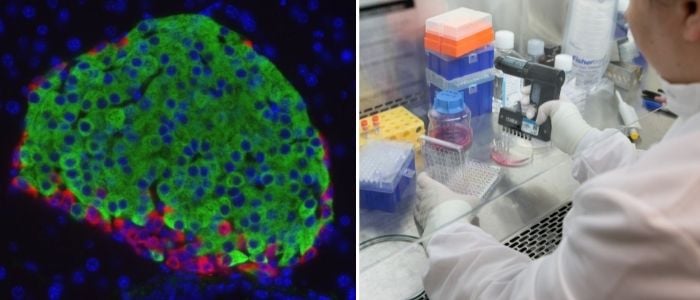
Dr. Harlan’s laboratory uses techniques they’ve adapted to precisely quantify the absolute and relative number of insulin-producing beta cells, and glucagon producing alpha cells found in donor islets, and the genes expressed by those cells. For instance, Sambra Redick, PhD, is developing techniques to remove and study single islets from slices of human pancreas received from the Network for Pancreatic Organ Donors with Diabetes (nPOD). Dr. Redick uses methods that allow her to obtain transcriptional profiles of single cells from within those islets.
Sally Kent, PhD, a UMass Chan Medical School DCOE immunologist characterizes the immune cells infiltrating the islets. Her lab is addressing key immunological, histological, viral and metabolic questions related to how T1D develops.
For this manuscript, the team described islets from a 22-year-old male who had been diagnosed with T1D eight years prior to his death. “Tissues generously donated by the families of deceased donors allow us to examine their islets to observe what their T-cells were doing at the time,” said Dr. Kent, associate professor of medicine and the George F. and Sybil H. Fuller Foundation Term Chair in Diabetes at UMass Chan Medical School DCOE.
“While these donated islets harbored many infiltrating T lymphocytes, many of the islets also had substantial numbers of beta cells, and the islets’ insulin secretory responses were remarkably intact, despite the patient’s poorly controlled diabetes,” added Dr. Harlan. “His islets also displayed quite abnormal glucagon responses.”
