Michael P. Czech, PhD
Michael P. Czech is The Isadore and Fannie Foxman Chair of Medical Research in the Program in Molecular Medicine at UMass Chan Medical School. He earned his PhD degree in biochemistry in 1972 at Brown University under the mentorship of Professor John Fain, and completed postdoctoral study in the Biochemistry Department at Duke University Medical Center. Dr. Czech moved to UMass Chan as Professor and Chair of the Department of Biochemistry in 1981 and served as the founding Chair of the Program in Molecular Medicine from 1989-2018. His research addresses mechanisms of signal transduction, metabolism and insulin resistance in type 2 diabetes and obesity. The Czech lab has applied RNAi and CRISPR techniques to discover novel drug targets and to develop therapeutic strategies for alleviating these metabolic diseases and nonalcoholic steatohepatitis.
Dr. Czech has served on several editorial boards and NIH Study Sections and is a member of the Scientific Review Board of the Howard Hughes Medical Institute. He has received the Scientific Achievement Award (1982), the Banting Medal (2000) and the Albert Renold Award for mentorship (2004) from the American Diabetes Association, the David Rumbough Scientific Award from JDRF (1985), NIH MERIT Awards (1997-2005 and 2012-2022) and the Elliot P. Joslin Medal (1998).
Major Area of Investigation
Gene editing & deletion to enhance insulin signaling and energy expenditure in type 2 diabetes and obesity
Central Questions for the Czech Lab
- Can we identify molecular mechanisms that disrupt insulin signaling in obesity and type 2 diabetes to develop therapeutic strategies for these diseases?
- Can we genetically modify adipocytes ex vivo with CRISPR technology to create cellular therapeutics for type 2 diabetes and its complications?
- Can we identify and modulate molecular mechanisms that switch adipocytes from storing triglyceride to cells that oxidize fat, expend energy, and secrete beneficial factors?
- Can we target genes with therapeutic oligonucleotides in order to enhance energy expenditure, reduce fat accumulation, and alleviate obesity and type 2 diabetes?
A complete list of Dr. Czech's published work can be found in My Bibliography
Current Research
Many of our projects utilize CRISPR and RNA interference (RNAi) to selectively silence normal or disease genes in vivo, providing powerful research tools & potential therapeutic approaches. Experiments in our lab are devoted to developing CRISPR- and siRNA-based delivery methods that can beneficially alter gene expression in adipocytes, hepatocytes and other cell types. Using these techniques, we have recently demonstrated that gene editing of adipocytes by CRISPR can enhance their energy expenditure and fat oxidation. Such genetically modified human adipocytes can cause weight loss and alleviate glucose intolerance when implanted in “humanized” mice (see Figure below). We have also shown that therapeutic siRNAs generated in the Khvorova and Watts laboratories have beneficial effects on liver metabolism in obese mice. These efforts are advancing toward therapeutic applications in cardiometabolic diseases.
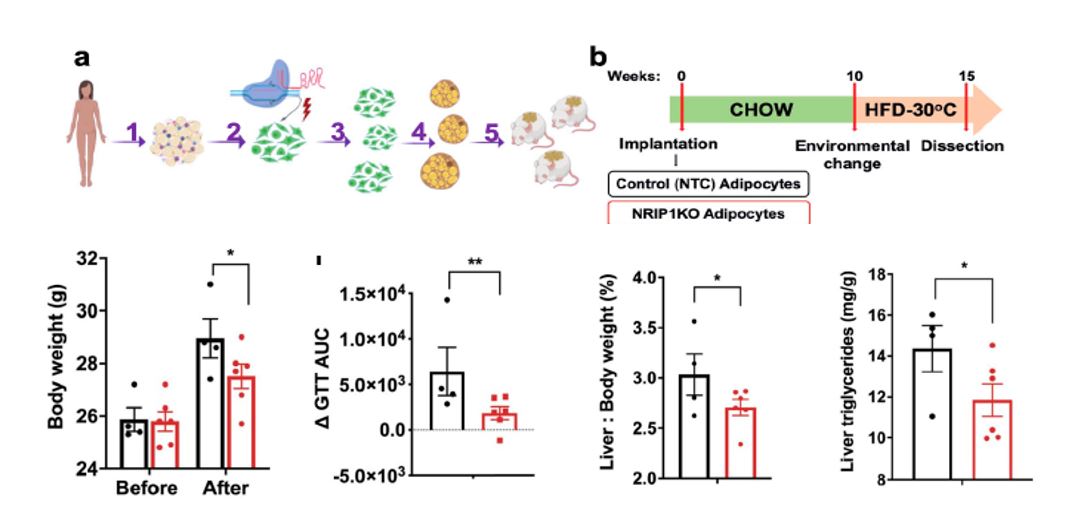
(Figure Description) Implantation of NRIP1/RIP140-targeted human adipocytes decreases body weight as well as liver triglyceride, and enhances glucose tolerance in recipient HFD fed NSG mice. Mice were implanted with either human adipocytes previously treated with NTC (non targeting control) sgRNA/Cas9 RNPs or with sgRNA-H5/Cas9 RNPs (NRIP1KO). a. Study description: (1) adipose tissue isolation from a human donor during panniculectomy, (2) harvesting of human primary preadipocytes after Cas9/sgRNA RNPs were delivered into the human preadipocytes by electroporation followed by (3) expansion 1:6 of the genetically modified preadipocytes and (4) their differentiation into mature adipocytes; (5) Implantation of non-targeted control (NTC) sgRNA treated adipocytes versus the NRIP1 depleted adipocytes was performed in the dorsal area of NSG mice. b. Schematic protocol of implantation of human NTC adipocytes or NRIP1KO adipocytes into NSG mice followed by HFD feeding. Lower panel bar graphs: Body weight, change in glucose tolerance test area under curve due to HFD feeding, liver to body weight ratio and liver triglyceride measurements in mice implanted with NTC human adipocytes (black bars) vs with Nrip1KO human adipocytes.
Contributions to Science
Identification of subunit configurations of the receptors for insulin and the insulin-like growth factors
Prior to the 1980s, there was an appreciation that receptor proteins for these peptides were present on cell surface membranes, but there was no biochemical information on these receptors. To approach this problem, Paul Pilch, then a postdoc in our lab, synthesized disuccinimidyl suberate as an affinity-labeling reagent for peptide hormone receptors and identified the two insulin receptor subunits, denoting these as alpha and beta in several publications. This approach was then used to deduce the disulfide-linked subunit configurations of the receptors for insulin and the insulin-like growth factors (IGF). This work clarified the overlapping binding affinities of insulin and the IGF ligands for these receptors and revealed that the IGF-2 receptor does not mediate the major bio-effects of these peptides. We showed the IGF-2 receptor is instead a target of insulin signaling, cloned the IGF-2 receptor in collaboration with Axel Ullrich and reported in Science it's identical to the mannose-6-phosphate receptor, i.e., it's a bi-functional receptor.
Pilch, P.F. and Czech, M.P. (1979). Interaction of Cross-Linking Agents with the Insulin Effector System of Isolated Fat Cells. Covalent Linkage of 125I-Insulin to a Plasma Membrane Receptor Protein of 140,000 Daltons. J. Biol. Chem., 254: 3375-3381.
Massague, J and Czech, M.P. (1982). The Subunit Structures of Two Distinct Receptors for Insulin-Like Growth Factors I and II and Their Relationship to the Insulin Receptor. J. Biol. Chem., 257: 5038-5045.
MacDonald. R.G., Pfeffer, S.R., Coussens, L., Tepper, M.A., Brocklebank, C.M., Mole, J.E., Anderson, J.K., Chen, E., Czech, M.P. and Ullrich, A. (1988) A Single Receptor Binds both Insulin-like Growth Factor II and Mannose-6-Phosphate. Science, 239: 1134-1137.
Identification of a key downstream target of insulin-stimulated PIP3 generation
As several groups identified PI 3-kinase as a component in insulin signaling, it became important to identify downstream signaling elements. Our group established an expression cloning screen for identifying targets of the PI 3-kinase pathway in insulin and IGF-1 receptor signaling, and discovered Grp1, a novel downstream effector of the signaling lipid PtdIns(3,4,5)P3 (PIP3). We reported in Science the identification of Grp1, showing that it defines a novel PI 3-kinase-mediated signaling pathway distinct from Akt, linking PIP3 to the activation of ArfGTPases. Jes Klarlund in our lab showed the Grp1 PH domain has the highest specificity for PIP3 of all the PH domains studied. The structural basis of selective PIP3 binding to the crystallized PH domain of Grp1 was solved in collaboration with David Lambright, published in Molecular Cell. The Grp1 PH domain fused to GFP is also now widely used by researchers as a unique reagent to define the generation of PIP3 at the plasma membrane.
Klarlund, J., Guilherme, A., Holik, J.J., Virbasius, J.V., Chawla, A., Czech, M.P. (1997). Signaling by3,4,5-Phosphoinositide through Proteins Containing Pleckstrin and Sec7 Homology Domains. Science, 275(5308):1927-30.
Lietzke, S.E., Bose, S., Cronin, T., Klarlund, J., Chawla, A., Czech, M.P., Lambright, D.G. (2000). Structural Basis of 3-Phosphoinositide Recognition by Pleckstrin Homology Domains. Molecular Cell, 6:385-394.
Identification of lipid droplet proteins and mechanisms of lipid storage
A focal point of our lab over many years has been the mechanisms that control triglyceride storage in adipocytes and their relationships to insulin resistance. We discovered a family of Cide-domain containing proteins is associated with lipid droplets in adipocytes and hepatocytes, and regulate lipid storage and turnover in these metabolic cell types. Gene deletion of Cidec/FSP27 and Cidea in mice has corroborated their key roles in lipid metabolism and whole body energy expenditure, and a human subject with a disrupting mutation in CIDEC displays lipodystrophy, insulin resistance and type 2 diabetes. We also found evidence that decreased lipid droplet protein expression in human adipose tissue may correlate with the appearance of insulin resistance, consistent with the hypothesis that they help sequester neutral lipids within adipocytes to protect other tissues from lipotoxicity.
Puri V, Konda S, Ranjit S, Aouadi M, Chawla A, Chouinard M, Chakladar A, Czech M.P. (2007) Fat- specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem., 282(47):34213-8..
Puri, V., Ranjit, S., Konda, S. Nicoloro, S.M., Straubhaar J, Chawla, A., Chouinard, M., Lin, C., Burkart A, Corvera, S., Perugini, R.A., Czech, M.P. (2008) Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Nat. Acad. Sci. USA, 105(22): 7833-7838.
Therapeutic strategies leveraging the power of siRNA and CRISPR technologies
Our laboratory has developed new modes of delivery of siRNA and CRISPR components to metabolic tissues for the purpose of targeting gene pathways in metabolic disease. We’ve used siRNA screening techniques to discover genes encoding metabolic suppressor activities, e.g., RIP140, that are now being advanced as potential therapeutic targets for obesity and type 2 diabetes. In collaboration with the Ostroff laboratory, vehicles for delivery of siRNAs to adipose tissue and liver macrophages were developed, and in collaboration with the Khvorova and Watts laboratories self-delivery, fully chemically modified siRNAs are being developed for metabolic tissue targeting. In addition, recent experiments using CRISPR techniques to disrupt RIP140 in human adipocytes in collaboration with the Corvera laboratory have yielded a promising strategy advancing toward cell therapeutic applications. Overall, these various interactive projects have leveraged the high potential of siRNA and CRISPR as potential future therapeutics for applications in obesity and type 2 diabetes.
Powelka AM, Seth, A, Virbasius, JV, Kiskinis, E, Nicoloro, SM, Tang, X, Straubhaar, J, Cherniack, AD, Parker, MG, Czech, M.P. (2006) Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. Journal of Clinical Investigation, 116(1):125-136.
Jourdan T, Godlewski G, Cinar R, Bertola A, Szanda G, Liu J, Tam J, Han T, Mukhopadhyay B, Skarulis MC, Ju C, Aouadi M, Czech M.P., Kunos G. (2013) Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nature Medicine, 19(9):1132-1140.
Yenilmez, B., Wetoska, N., Kelly, M., Echeverria, D., Min, K., Lifshitz, L., Alterman, J.F., Hassler, M.R., Hildebrand, S., DiMarzio, C., McHugh, N., Vaneieli, L., Sousa, J., Pan, M., HanX., Brehm, M.A., Khvorova, A., Czech, MP. (2022) An RNAi therapeutic targeting hepatic DGAT2 in a genetically obese mouse model of nonalcoholic steatohepatitis. Molecular Therapy, 30 (3), 1329-1342
Tsagkaraki, E, Nicoloro, S.M., DeSouza, T., Solivan-Rivera, J., Desai, A., Lifshitz, L.M., Shen, Y., Kelly, M., Guilherme, A., Henriques, F.,et al Czech, M.P., (2021) CRISPR-enhanced human adipocyte browning as cell therapy for metabolic disease. Nature Communications, 12 (1):6931-6947
Graduate Student Rotation & Thesis Research Projects
Developing CRISPR technology for gene editing in adipocytes
Adipocytes function as master regulators of whole body metabolism and deletions of specific genes in knockout mice promote dramatic improvements in glucose tolerance and insulin sensitivity in diabetic models. Thus the ability to direct gene editing in adipocytes in vivo has great potential in developing therapeutic strategies for obesity, type 2 diabetes and cardiovascular complications. Alternatively, an ex vivo approach is also feasible since adipose cells can be genetically altered in vitro prior to implantation into mice with beneficial effects. Such projects are designed to be performed in collaboration with other Czech lab members with the goal of specifically deleting or editing genes in adipose depots that drive increases in fatty acid oxidation, energy expenditure and systemic metabolic activity. Exciting preliminary data at the “proof of principle” stage has been achieved and this project is designed to focus on specific genes of high interest for therapeutics.
Mentoring
The Czech lab actively mentors graduate students and postdoctoral fellows. Many former students and postdoc trainees from our lab are now internationally recognized professors at major universities and medical schools. They include:
Paul Pilch, Professor of Biochemistry, Boston University School of Medicine
Jeffrey Pessin, Professor and Director of the Diabetes Center, Einstein College of Medicine
Joan Massague, Director, Sloan Kettering Institute
Roger Davis, HHMI investigator and Professor of Molecular Medicine, UMass Chan Medical School
Silvia Corvera, Professor of Molecular Medicine, UMass Chan Medical School
Carla Greenbaum, Vice Chair and Director of Clinical Research, Benaroya Research Institute
Jes Klarlund, Professor of Ophthalmology, University of Pittsburgh Medical Center
Rob Lewis, Professor of Biochemistry and Molecular Biology, Eppley Cancer Institute, University of Nebraska Medical Center
Richard MacDonald, Professor of Biochemistry and Molecular Biology, Eppley Cancer Institute, University of Nebraska Medical Center
Assia Shisheva, Professor of Physiology, Wayne State School of Medicine.
Mark Sleeman, Professor of Physiology, Monash University, Australia
John Harris, Associate Professor of Medicine, UMass Chan Medical School
Zhen Jiang, Associate Professor of Pharmacology and Medicine, Boston University
Vishu Puri, Professor of Biomedical Sciences, Ohio University
Olga Gupta, Assistant Professor of Pediatrics and Medicine, University of Texas Southwestern Medical Center
Tim Fitzgibbons, Assistant Professor of Medicine, UMass Chan Medical School
Myriam Aouadi, Assistant Professor, Integrated Cardio Metabolic Centre, Karolinska Institute
Adilson Guilherme, Associate Professor of Molecular Medicine, UMass Chan Medical School
Control of Adipocyte Thermogenesis and Lipogenesis through β3-Adrenergic and Thyroid Hormone Signal Integration

Type 2 diabetes research on behavior and function of the various cells that make up adipose tissue, with a goal of improving systemic metabolism in obesity. We recently revealed new insights into signaling that drives heat production in beige adipocytes. Full article in Cell Reports
Felipe Henriques led a study published in Cell Reports on white adipose beiging

"Single Cell RNA Profiling Reveals Adipocyte to Macrophage Signaling Sufficient to Enhance Thermogenesis" Full Article
Type 2 diabetes research studying the behavior and function of the cells that make up adipose tissue

Type 2 diabetes research in the laboratory of Michael Czech, PhD, includes studying the behavior and function of the various cells that make up adipose tissue, with a goal of improving systemic metabolism in obesity. They contributed some of their RNAi technology to a study published in Nature Medicine.
$2.5M grant to advance potential therapy for T2D

Michael Czech, PhD, is collaborating with Silvia Corvera, MD to investigate whether technologies developed in their labs will harness beige fat’s ability to burn energy and accelerate metabolism in order to improve the body’s response to sugar and lower blood glucose levels. Learn More

