Research
The cJun NH2-terminal kinase (JNK) is is a member of the MAP kinase group that is activated by exposure to physical/chemical stress and by many chemokines, cytokines, and growth factors. JNK is encoded by three genes (Mapk8, Mapk9, and Mapk10) that encode the JNK1, JNK2, and JNK3 protein kinases. Alternative pre-mRNA splicing results in the expression of ten JNK isoforms that exhibit differences in substrate specificity.
The goal of the Davis laboratory research program is to understand the molecular mechanism and biological significance of JNK signaling. We use a combination of experimental approaches to examine JNK signaling, including mouse models and human studies.
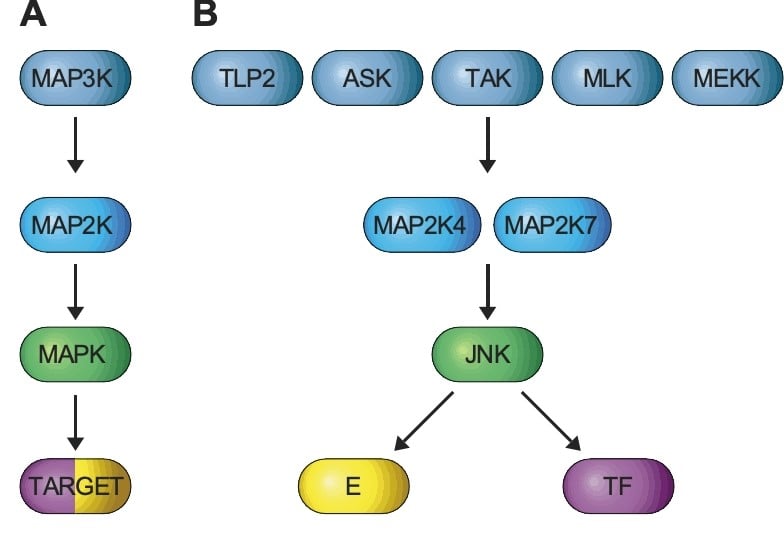
The JNK signaling Pathway
A) JNK is activated by a three-tiered kinase cascade formed by a MAPK, a MAP2K, and a MAP3K. B) Groups of MAP3K (e.g. TPL2, ASK, TAK, MLK, and MEKK) phosphorylate and activate two MAP2K (MAP2K4 and MAP2K7) that phosphorylate and activate JNK leading to the phosphorylation of protein substrates (TARGETS), including enzymes (E) and transcription factors (TF).
Highlights of Recent Research by the Davis Laboratory
JNK and metabolic syndrome
JNK is activated in response to metabolic stress and promotes obesity, chronic inflammation, and insulin resistance. These physiological processes are mediated by functions of JNK within different tissues. For example, obesity of regulated by a hypothalamic role of JNK that regulates energy expenditure (JNK1 & JNK2) and feeding behavior (JNK3). Furthermore, JNK controls M1 macrophage differentiation and therefore promotes inflammation. Moreover, the effect of JNK to promote insulin resistance in peripheral tissues is largely a consequence of the effects of JNK to regulate fat metabolism by suppressing signaling by PPAR nuclear hormone receptors.
The molecular mechanisms that mediate JNK activation in response to metabolic stress, the identification of specific JNK isoforms that mediate the effects of metabolic stress, and the molecular targets of metabolic stress-induced JNK signaling remain to be fully established. These questions are a current focus of the Davis laboratory.
JNK and metabolism
JNK promotes hepatic steatosis by suppressing the activity of the PPARa nuclear hormone receptor.
JNK and cancer
JNK is implicated in the etiology of cancer. However, the role of JNK is complex. Activated JNK can promote cell death; for example during the detachment of epithelial cells (anoikis) and in response to chemotherapeutic agents. In contrast, activated JNK can also promote proliferation. The decision to follow either of these cell fates is defined by the network status of signaling pathways within the cell. A systems biology approach is key to understanding JNK function. Moreover, these considerations have identified paradigms for potential combination therapy.
Recent studies by the Davis laboratory have focused on breast cancer. These studies have demonstrated that JNK plays a major role in the regulation of genome stability and that JNK pathway mutations can act as drivers for tumor development. The molecular mechanism of JNK-regulated genome stability is poorly defined. This represents a major focus of current research by the Davis laboratory.
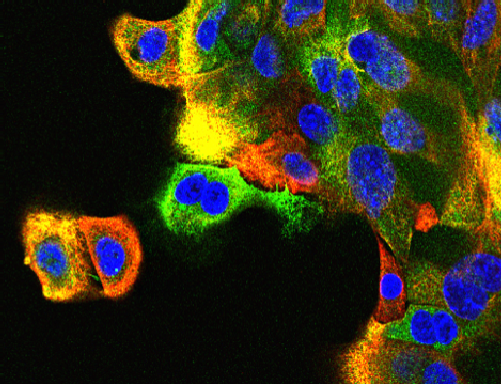
JNK and breast cancer
JNK supression mammary tumor formation. Breast tumor cells isolated from mice with compound JNK-deficiency in the mammary epithelium were stained with antibodies to cytokeratin 5 (red) and cytokeratin 8 (green). DNA was stained with DAPI.
Summary
The overall goal of the research in the Davis laboratory is to gain a molecular understanding the function of JNK during normal physiology and disease. This knowledge is essential because it provides the foundation for the design of novel therapeutic strategies for the successful treatment of human disease.