Tay-Sachs Disease
Gene therapy program for rare genetic disease
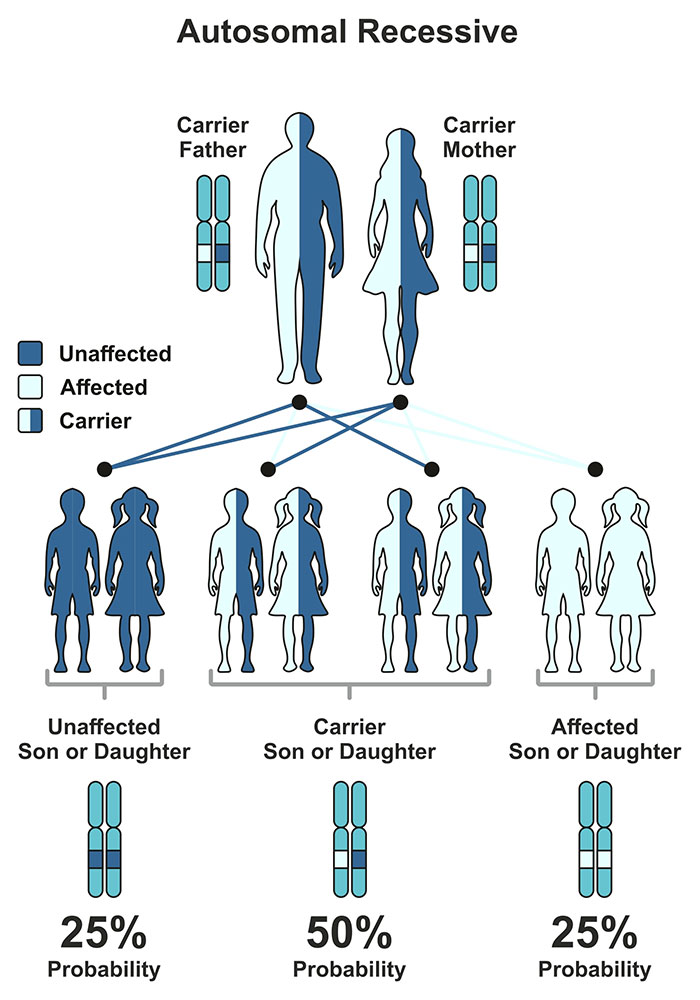 Tay-Sachs disease (TSD) is a recessive lysosomal storage disorder that results in progressive impairment of neurologic function. Dozens of lysosomal storage disorders (LSDs) have been identified, all of which result in the inability of the body to rid itself of certain waste products and consequent accumulation of these waste products within the cell. Approximately 75 percent of all LSDs cause damage to the nervous system. With few exceptions, the neurologic LSDs (such as Tay-Sachs disease) are fatal and untreatable. Tay-Sachs is caused by an enzymatic deficiency of the enzyme β-N-acetyl hexosaminidase, which exists in three separate forms: HexA, HexB and HexS. HexA is composed of α and β subunits. Tay-Sachs disease is the result of a loss of function of the α subunit of HexA.
Tay-Sachs disease (TSD) is a recessive lysosomal storage disorder that results in progressive impairment of neurologic function. Dozens of lysosomal storage disorders (LSDs) have been identified, all of which result in the inability of the body to rid itself of certain waste products and consequent accumulation of these waste products within the cell. Approximately 75 percent of all LSDs cause damage to the nervous system. With few exceptions, the neurologic LSDs (such as Tay-Sachs disease) are fatal and untreatable. Tay-Sachs is caused by an enzymatic deficiency of the enzyme β-N-acetyl hexosaminidase, which exists in three separate forms: HexA, HexB and HexS. HexA is composed of α and β subunits. Tay-Sachs disease is the result of a loss of function of the α subunit of HexA.
TSD presents in three forms: infantile, juvenile onset and adult onset. By age two, children with the infantile form suffer from seizures, swallowing difficulties, respiratory infections and loss of motor control. The disease is fatal by age three to five. In the juvenile form, children are often diagnosed in kindergarten at five to seven years of age. These children experience speech abnormalities (stuttering), learning difficulties and clumsiness. They become spastic, lose the ability to walk, talk and eat, and develop seizures. Eventually these individuals reach a vegetative state, with death occurring in the second decade of life. The adult onset form is not fatal, but results in significant disability, such as peripheral neuropathy, speech impairment and loss of mobility. No accepted treatment currently exists for TSD.
A clinically significant animal model of TSD was first reported in 2010 in Jacob sheep. The TSD sheep develop a variety of clinical manifestations of the disease, which has allowed our lab to track the efficacy of novel therapeutics. Neurological assessments, behavioral testing and MRI are some of the tools our lab has utilized to understand disease progression.
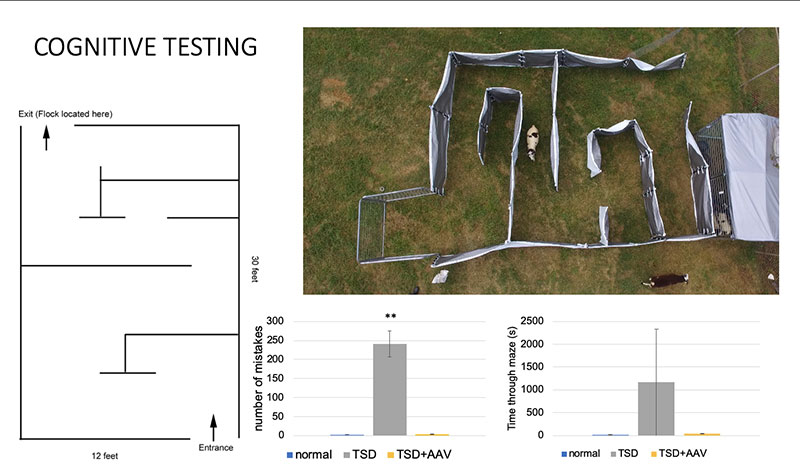
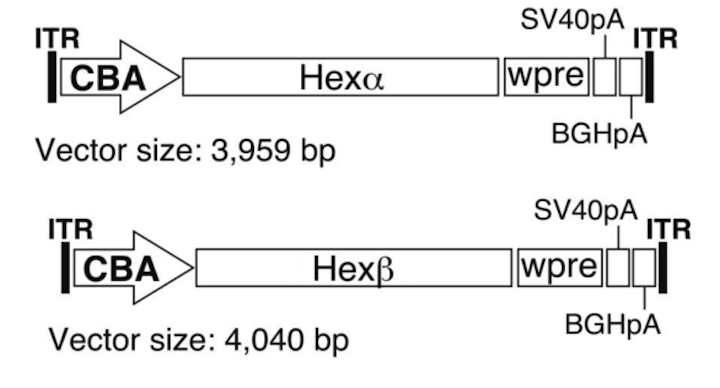 In conjunction with our collaborators Miguel Sena-Esteves, PhD, at UMass Chan and Doug Martin, PhD, at Auburn University AAV gene therapy studies were conducted that tested co-injection of two separate AAV vectors encoding both HexA subunits separately (α+β) via multiple delivery routes. AAV is a small virus that infects the host without causing disease, so it can be used to deliver a gene with minimal risk of adverse effects.These studies showed significant improvement in disease progression in TSD sheep. Based on these and other studies, compassionate use trials were conducted that treated two children with the infantile form of TSD. During preclinical development and progress toward clinical translation, we have worked closely with foundations including the Blue Genes Foundation, the National Tay-Sachs and Allied Diseases Foundation (NTSAD), and the Cure Tay-Sachs Foundation (CTSF). The TSD gene therapy program has since been licensed by Axovant Gene Therapies, with Phase I/II clinical trials upcoming.
In conjunction with our collaborators Miguel Sena-Esteves, PhD, at UMass Chan and Doug Martin, PhD, at Auburn University AAV gene therapy studies were conducted that tested co-injection of two separate AAV vectors encoding both HexA subunits separately (α+β) via multiple delivery routes. AAV is a small virus that infects the host without causing disease, so it can be used to deliver a gene with minimal risk of adverse effects.These studies showed significant improvement in disease progression in TSD sheep. Based on these and other studies, compassionate use trials were conducted that treated two children with the infantile form of TSD. During preclinical development and progress toward clinical translation, we have worked closely with foundations including the Blue Genes Foundation, the National Tay-Sachs and Allied Diseases Foundation (NTSAD), and the Cure Tay-Sachs Foundation (CTSF). The TSD gene therapy program has since been licensed by Axovant Gene Therapies, with Phase I/II clinical trials upcoming.
Our lab is continuing our studies of TSD in sheep, with a flock of more than 100 sheep currently housed at the Tufts Cummings School of Veterinary Medicine in Grafton, Mass.
To contact us about TSD, please email Dr. Toloo Taghian at toloo.taghian@umassmed.edu